Contents
4 . Order Entry: General, Laboratory, Dietary, Supply, Blood Transfusion
Chapter Chair:
Hans Buitendijk Cerner Corporation
Chapter Chair:
Robert Hausam MD Hausam Consulting
Chapter Chair:
Patrick Loyd ICode Solutions
Chapter Chair
Ken McCaslin Rene Harvey and Associates
Chapter Chair
Lorraine Constable HL7 Canada
Chapter Chair
Riki Merrick Vernetzt, LLC
Editor
Brian Pech Kaiser Permanente
Sponsoring Committee:
Orders & Observations
List Server:
ord@lists.hl7.org
4.1 CHAPTER 4 CONTENTS
4.2 PURPOSE
The Order Entry transaction set provides for the transmission of orders or information about orders between applications that capture the order, by those that fulfill the order, and other applications as needed. An order is a request for material or services, usually for a specific patient. These services include medications from the pharmacy, clinical observations (e.g., vitals, I&Os) from the nursing service, tests in the laboratory, food from dietary, films from radiology, linens from housekeeping, supplies from central supply, an order to give a medication (as opposed to delivering it to the ward), etc.
Most orders are associated with a particular patient. However, the Standard also allows a department to order from another ancillary department without regard to a patient (e.g., floor stock), as well as orders originating in an ancillary department (i.e., any application may be the placer of an order or the filler of an order).
We refer to the person or entity who places the order as the placer. We refer to the person or entity that carries out the order as the filler (producer in ASTM terminology). In the case where the person or entity that carries out the order also requests the order, this person or entity is referred to as the filler and placer of the order. The filler may also request another application to assign a filler or placer order number.
This chapter defines the transactions at the seventh level, i.e., the abstract messages. Various schemes may be used to generate the actual characters that make up the messages according to the communications environment. The HL7 Encoding Rules will be used where there is not a complete Presentation Layer. This is described in Chapter 2, Section 2.6, "Message construction rules." The examples included in this chapter were constructed according to the HL7 Encoding Rules.
4.2.1 Preface (organization of this chapter)
This chapter has been organized into six major sections, General, Diet, Supply, Pharmacy, Vaccine and Transfusion Services. Each section contains the trigger events, message definitions, segments and examples for the specific type of order messages. Each section about a type of order is organized into background and overview, message structure, and message segments (that are specific to the order class in question). Special discussions of the use of fields, segments or messages, and examples are included. Segments are introduced in order of occurrence in a message. A list of allowable values for a field is included in the body of the text, along with the field definition for easier reference.
Section 4.3 refers the reader to Chapter 2 for an outline of the Quantity Timing (TQ) Data Type Definition.
Sections 4.4 to 4.6 'General' includes the triggers and segments for the clinical observations and diagnostic studies as well as the triggers and message segments that are common to all of the order entry messages. Orders for laboratory tests, bedside monitoring, diagnostic imaging, electrocardiograms, vital signs, etc., are subsumed under this order message set.
Sections 4.7 to 4.9 'Diet' includes all of the usual diet specifications including snacks and guest trays
Sections 4.10 to 4.12 'Supply' includes order messages for both Stock and No-stock orders. Supply orders are different in that they often are not patient-centered (e.g., requests to stock the ward supply room).
Sections 4.13 to 4.16 'Pharmacy / Treatment' includes all pharmacy and treatment related order messages. These sections additionally include triggers related to the dispensing, giving and administration of orders. In the development of the treatment order transaction set, the focus has been on medication treatments, but the same transaction set works well for total parenteral nutrition (TPN). There is hope that it is also sufficient for other kinds of treatment orders, such as those performed by the nursing service. But it has not yet been exercised in that context and may well need further development.
Sections 4.17 to 4.19 'Vaccine' includes triggers and segments specific to vaccination order messages. These sections also include RXA definitions specific to vaccination messages.
Sections 4.20 to 4.22 "Transfusion Service (Blood Bank)" includes triggers and segments specific to transfusion service messages.
4.2.2 Glossary
4.2.2.0
4.2.2.1 Filler:
The application responding to, i.e., performing, a request for services (orders) or producing an observation. The filler can also originate requests for services (new orders), add additional services to existing orders, replace existing orders, put an order on hold, discontinue an order, release a held order, or cancel existing orders
4.2.2.2 Observation segment:
An OBX segment defined in Chapter 7.
4.2.2.3 Order:
A request for a service from one application to a second application. The second application may in some cases be the same, i.e., an application is allowed to place orders with itself. In HL7 terms, an order is defined as an ORC segment in conjunction with a single order detail segment such as OBR, RXO or RXE.
4.2.2.4 Order detail segment:
One of several segments that can carry order information. Examples are OBR and RXO. Future ancillary-specific segments may be defined in subsequent releases of the Standard if they become necessary.
4.2.2.5 Placer:
The application or individual originating a request for services (order).
4.2.2.6 Placer order group:
A list of associated orders coming from a single location regarding a single patient.
4.2.2.7 Order Number:
An identifier that uniquely identifies an order as represented by an ORC segment and its matching order detail segment. Although traditionally called an order number, the identifier is not required to be all digits, it may contain alpha as well as numeric characters.
Examples:
Example 1
|
Order Number
|
Group Number
|
Parent
|
Example 2
|
Order Number
|
Group Number
|
Example 3
Note: With version 2.5, the definition and narrative for the TQ - Quantity/Timing data type has been moved to Chapter 2, Section 2.A.81. This section retained in v2.6 and later to maintain consistent section numbering for reference from other chapters.
4.4 GENERAL TRIGGER EVENTS & MESSAGE DEFINITIONS
This section includes trigger events and message definitions that are general to all orders in addition to the Observation and Diagnostic Study.
4.4.1 ORM - general order message
Attention: Retained for backwards compatibility only as of v2.4.and withdrawn as of v2.7. Refer to OMG, OML, OMD, OMS, OMN, OMI, and OMP instead.
4.4.2 ORR - general order response message response to any ORM
Attention: Retained for backwards compatibility only as of v2.5 and withdrawn as of v2.7. Refer to ORG, ORL, ORD, ORS, ORN, ORI, and ORP instead.
4.4.3 OSQ/OSR- query response for order
Attention: Retained for backwards compatibility only as of v2.4.and withdrawn as of v2.7. Refer to Chapter 5.
4.4.4 OMG - general clinical order message (event O19)
The function of this message is to initiate the transmission of information about a general clinical order that uses the OBR segment. OMG messages can originate also with a placer, filler, or an interested third party.
The trigger event for this message is any change to a general clinical order. Such changes include submission of new orders, cancellations, updates, patient and non-patient-specific orders, etc.
This trigger includes segments identified as being for 'previous results.' These segments allow the sending system to include demographic and/or result information from previous result reports when they are related to the current order.
For example:
-
Diagnostic laboratories referring tests to another lab for either confirmation of results (HIV, etc.) or due to not being equipped to do the tests (genetic testing, etc.).
-
Diagnostic laboratories sending test results to Knowledge Bases for the automated generation of diagnostic comments for inclusion into the lab report.
The CTD segment in this trigger is used to transmit temporary patient contact details specific to this order.
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OMG^O19^OMG_O19: General Clinical Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
OBR
|
Observation
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data
|
|
11
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[{
|
--- CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- CONTAINER_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Container Observation)
|
|
7
|
DB |
|
}]
|
--- CONTAINER_OBSERVATION end
|
|
|
|
|
}]
|
--- CONTAINER end
|
|
|
|
|
}]
|
--- SPECIMEN end
|
|
|
|
|
[
SGH
]
|
Segment Group Header
|
|
2
|
DB |
|
[{
|
--- PRIOR_RESULT begin
|
|
|
|
|
[
|
--- PATIENT_PRIOR begin
|
|
|
|
|
PID
|
Patient Identification - previous result
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics - previous result
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit - previous result
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit Add. Info - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information - previous result
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
ORC
|
Common Order - previous result
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Order Detail - previous result
|
|
4
|
DB |
|
[{
|
--- TIMING_PRIOR begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_PRIOR end
|
|
|
|
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior) - previous result
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data - previous result
|
|
10
|
DB |
|
{
|
--- OBSERVATION_PRIOR begin
|
|
|
|
|
OBX
|
Observation/Result - previous result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Oservation Prior)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
}
|
--- OBSERVATION_PRIOR end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
}]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
SGT
]
|
Segment Group Trailer
|
|
2
|
DB |
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.4.5 ORG - general clinical order acknowledgement message (event O20)
The function of this message is to respond to an OMG message. An ORG message is the application acknowledgment to an OMG message. See Chapter 2 for a description of the acknowledgment paradigm.
In ORG the PID and ORC segments are optional, particularly in case of an error response. However, ORC segments are always required in ORG when the OBR is present. For example, a response ORG might include only the MSH and MSA.
The function (e.g., cancel, new order) of both OMG and ORG messages is determined by the value in ORC-1-order control. (See the table of order control values for a complete list.)
ORG^O20^ORG_O20: General Clinical Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_GROUP begin
|
|
|
|
|
OBR
|
Observation
|
|
4
|
DB |
|
]
|
--- OBSERVATION_GROUP end
|
|
|
|
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[
{
SAC
}
]
|
Specimen Container Details
|
|
13
|
DB |
|
}]
|
--- SPECIMEN end
|
|
|
|
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.6 OML - laboratory order message (event O21)
The following message structure may be used for the communication of laboratory and other order messages and must be used for lab automation messages where it is required that the Specimen/Container information is within the ORC/OBR segment group.
The trigger event for this message is any change to a laboratory order. Such changes include submission of new orders, cancellations, updates, etc. OML messages can originate also with a placer, filler, or an interested third party.
Note: The additional patient information, which is sent after the OBR with the current order (the segments PID, PD1, PV1, PV2, etc, indicated below with words "previous result"), could have been transferred with the previous result because the patient demographics related to the previous result can differ from the demographics related to the current order. The current intent is to only allow references to the same patient as in the header PID.
The SAC segments included in the message allow the transfer of, e.g., a laboratory order with multiple containers and multiple test orders related to each container, or laboratory orders with test order requiring multiple containers.
Refer to Chapter 13, "Laboratory Automation" for examples of usage, particularly to clarify the use of two references to SAC segments in this one message.
The CTD segment in this trigger is used to transmit temporary patient contact details specific to this order.
In relationship to triggers O21, O33, O35, and O39 this message/trigger (O21) should be used where an order with multiple samples and optionally multiple containers per order item are to be communicated, but not against a complete specimen shipment (O39)
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OML^O21^OML_O21: Laboratory Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
[{
|
--- TIIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
TCD
]
|
Test Code Details
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data
|
|
11
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for OBX)
|
|
7
|
DB |
|
[
TCD
]
|
Test Code Detail
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[{
|
--- CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- CONTAINER_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result related to container
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Container Observation)
|
|
7
|
DB |
|
}]
|
--- CONTAINER_OBSERVATION end
|
|
|
|
|
}]
|
--- CONTAINER end
|
|
|
|
|
}]
|
--- SPECIMEN end
|
|
|
|
|
[
SGH
]
|
Segment Group Header
|
|
2
|
DB |
|
[{
|
--- PRIOR_RESULT begin
|
|
|
|
|
[
|
--- PATIENT_PRIOR begin
|
|
|
|
|
PID
|
Patient Identification - previous result
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit - previous result
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit Add. Info - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information - previous result
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
ORC
|
Common Order - previous result
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Order Detail - previous result
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior)
|
|
7
|
DB |
|
[{
|
--- TIMING_PRIOR begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_PRIOR end
|
|
|
|
|
{
|
--- OBSERVATION_PRIOR begin
|
|
|
|
|
OBX
|
Observation/Result - previous result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Prior)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
}
|
--- OBSERVATION_PRIOR end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
}]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
SGT
]
|
Segment Group Trailer
|
|
2
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.4.7 ORL - general laboratory order response message to any OML (event O22)
The function of this message is to respond to an OML message. An ORL message is the application acknowledgment to an OML message. See Chapter 2 for a description of the acknowledgment paradigm.
Two message structures are available to acknowledge OML_O21:
With patient segments
Optionally without patient segments
4.4.7.1 Patient Segments Required
ORL^O22^ORL_O22: General Laboratory Order Acknowledgment Message (Patient Required)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[
{
SAC
}
]
|
Specimen Container Details
|
|
13
|
DB |
|
}]
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.7.2 Patient Segments Optional
ORL^O22^ORL_O41: General Laboratory Order Acknowledgment Message (Patient Optional)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[
{
SAC
}
]
|
Specimen Container Details
|
|
13
|
DB |
|
}]
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.8 OML - Laboratory order for multiple orders related to a single specimen (event O33)
The trigger event for this message is any change to a laboratory order. Such changes include submission of new orders, cancellations, updates, etc., where multiple orders are associated with a single sample which may be carried in multiple containers. OML messages can originate also with a placer, filler, or an interested third party.
This allows for a Specimen-centric message with multiple orders per specimen grouped by the specimen.
The following message structure may be used for the communication of laboratory and other order messages and must be used for lab automation messages where the message requires Specimen/container information to group a number of orders.
In relationship to triggers O21, O33, and O35, this message/trigger (O33) should be used where a specimen, with optional multiple containers, may have multiple orders to be communicated.
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OML^O33^OML_O33: Laboratory Order - Multiple Order Per Specimen Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[
{
SAC
}
]
|
Specimen Container
|
|
13
|
DB |
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
TCD
]
|
Test Code Details
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
TCD
]
|
Test Code Detail
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[
SGH
]
|
Segment Group Header
|
|
2
|
DB |
|
[{
|
--- PRIOR_RESULT begin
|
|
|
|
|
[
|
--- PATIENT_PRIOR begin
|
|
|
|
|
PID
|
Patient Identification - previous result
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit - previous result
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit Add. Info - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information - previous result
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
ORC
|
Common Order - previous result
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Order Detail - previous result
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior)
|
|
7
|
DB |
|
[{
|
--- TIMING_PRIOR begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_PRIOR end
|
|
|
|
|
{
|
--- OBSERVATION_PRIOR begin
|
|
|
|
|
OBX
|
Observation/Result - previous result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Prior)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
}
|
--- OBSERVATION_PRIOR end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
}]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
SGT
]
|
Segment Group Trailer
|
|
2
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
4.4.9 ORL - Laboratory order response message to a multiple order related to single specimen OML (event O34)
The function of this message is to respond to an OML message where the original trigger event produced an OML with the Specimen Group segment above the ORC. An ORL message is the application acknowledgment to an OML message. See Chapter 2 for a description of the acknowledgment paradigm.
Two message structures are available to acknowledge OML_O34:
With patient segments
Optionally without patient segments
4.4.9.1 Patient Segments Required
ORL^O34^ORL_O34: Laboratory Order Acknowledgment Message - Multiple Order Per Specimen (Patient Required)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN OBSERVATION end
|
|
|
|
|
[
{
SAC
}
]
|
Specimen Container
|
|
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.9.2 Patient Segments Optional
ORL^O34^ORL_O42: Laboratory Order Acknowledgment Message - Multiple Order Per Specimen (Patient Optional)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN OBSERVATION end
|
|
|
|
|
[
{
SAC
}
]
|
Specimen Container
|
|
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.10 OML - Laboratory order for multiple orders related to a single container of a specimen (event O35)
The trigger event for this message is any change to a laboratory order. Such changes include submission of new orders, cancellations, updates, etc., where multiple orders are associated with a single sample which may be carried in multiple containers. OML messages can originate also with a placer, filler, or an interested third party.
This allows for a Specimen-centric message with multiple orders per specimen grouped by the specimen.
The following message structure may be used for the communication of laboratory and other order messages and must be used for lab automation messages where the message requires Specimen/container information to group a number of orders.
In relationship to triggers O21, O33, and O35, this message/trigger (O35) should be used for laboratory orders where there is 1 or more Specimens with 1 to many containers and each container may have 1 to many orders with previous result(s) per container.
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OML^O35^OML_O35: Laboratory Order - Multiple Order Per Container of Specimen Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Speciment Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
{
|
--- SPECIMEN_CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
TCD
]
|
Test Code Details
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
TCD
]
|
Test Code Detail
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[
SGH
]
|
Segment Group Header
|
|
2
|
DB |
|
[{
|
--- PRIOR_RESULT begin
|
|
|
|
|
[
|
--- PATIENT_PRIOR begin
|
|
|
|
|
PID
|
Patient Identification - previous result
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit - previous result
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit Add. Info - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information - previous result
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
ORC
|
Common Order - previous result
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Order Detail - previous result
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior)
|
|
7
|
DB |
|
[{
|
--- TIMING_PRIOR begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_PRIOR end
|
|
|
|
|
{
|
--- OBSERVATION_PRIOR begin
|
|
|
|
|
OBX
|
Observation/Result - previous result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Prior)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
}
|
--- OBSERVATION_PRIOR end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
}]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
SGT
]
|
Segment Group Trailer
|
|
2
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN_CONTAINER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
4.4.11 ORL - Laboratory order response message to a single container of a specimen OML (event O36)
The function of this message is to respond to an OML message where the original trigger event produced an OML with the Specimen Group segment above the ORC. An ORL message is the application acknowledgment to an OML message. See Chapter 2 for a description of the acknowledgment paradigm.
Two message structures are available to acknowledge OML_O36:
With patient segments
Optionally without patient segments
4.4.11.1 Patient Segments Required
ORL^O36^ORL_O36: Laboratory Order Acknowledgment Message - Multiple Order Per Container of Specimen (Patient Required)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Related Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN OBSERVATION end
|
|
|
|
|
[
{
NTE
}
]
|
Notes and Comments (for specimen)
|
|
2
|
DB |
|
{
|
--- SPECIMEN_CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN_CONTAINER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.11.2 Patient Segments Optional
ORL^O36^ORL_O43: Laboratory Order Acknowledgment Message - Multiple Order Per Container of Specimen (Patient Optional)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Related Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN OBSERVATION end
|
|
|
|
|
[
{
NTE
}
]
|
Notes and Comments (for specimen)
|
|
2
|
DB |
|
{
|
--- SPECIMEN_CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
}
|
--- SPECIMEN_CONTAINER end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.12 OML - Specimen shipment centric laboratory order (event O39)
The function of this message is to apply an order to all specimens in a shipment or a package within a shipment..
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OML^O39^OML_O39: Specimen Shipment Centric Laboratory Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
TCD
]
|
Test Code Details
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data
|
|
11
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
TCD
]
|
Test Code Detail
|
|
13
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[{
|
--- SPECIMEN_SHIPMENT begin
|
|
|
|
|
SHP
|
Shipment Segment
|
|
|
DB |
|
[{
|
--- SHIPMENT_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result Segment (Additional Shipping Information)
|
|
|
DB |
|
[
{
PRT
}
]
|
Participation (for OBX)
|
|
7
|
DB |
|
}]
|
--- SHIPMENT_OBSERVATION end
|
|
|
|
|
{
|
--- PACKAGE begin
|
|
|
|
|
PAC
|
Shipping Package Segment
|
|
|
DB |
|
[{
|
--- SPECIMEN_IN_PACKAGE begin
|
|
|
|
|
SPM
|
Specimen Information
|
|
13.4.3
|
DB |
|
[{
|
--- SPECIMEN OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result Segment (For Specimen)
|
|
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[{
|
--- SPECIMEN_CONTAINER_IN_PACKAGE begin
|
|
|
|
|
SAC
|
Container Information
|
|
7.4.3
|
DB |
|
[{
|
--- CONTAINER_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result Segment (For Container)
|
|
|
DB |
|
[
{
PRT
}
]
|
Participation (for Container Observation)
|
|
7
|
DB |
|
}]
|
--- CONTAINER_OBSERVATION end
|
|
|
|
|
}]
|
--- SPECIMEN_CONTAINER_IN_PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_IN_PACKAGE end
|
|
|
|
|
}
|
--- PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_SHIPMENT end
|
|
|
|
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.4.13 ORL - Specimen shipment centric laboratory order response message to specimen shipment OML (event O40)
The function of this message is to respond to an OML message. An ORL message is the application acknowledgment to an OML message. See Chapter 2 for a description of the acknowledgment paradigm.
Two message structures are available to acknowledge OML_O40:
With patient segments
Optionally without patient segments
4.4.13.1 Patient Segments Required
ORL^O40^ORL_O40: Specimen Shipment Centric Laboratory Order Acknowledgment Message (Patient Required)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_SHIPMENT begin
|
|
|
|
|
SHP
|
Shipment Segment
|
|
|
DB |
|
{
|
--- PACKAGE begin
|
|
|
|
|
PAC
|
Shipping Package Segment
|
|
|
DB |
|
[{
|
--- SPECIMEN_IN_PACKAGE begin
|
|
|
|
|
SPM
|
Specimen Information
|
|
13.4.3
|
DB |
|
[{
|
--- SPECIMEN_CONTAINER_IN_PACKAGE begin
|
|
|
|
|
SAC
|
Container Information
|
|
7.4.3
|
DB |
|
}]
|
--- SPECIMEN_CONTAINER_IN_PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_IN_PACKAGE end
|
|
|
|
|
}
|
--- PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_SHIPMENT end
|
|
|
|
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- PATIENT end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.13.2 Patient Segments Optional
ORL^O40^ORL_O44: Specimen Shipment Centric Laboratory Order Acknowledgment Message (Patient Optional)
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
]
|
|
|
|
|
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_SHIPMENT begin
|
|
|
|
|
SHP
|
Shipment Segment
|
|
|
DB |
|
{
|
--- PACKAGE Begin
|
|
|
|
|
PAC
|
Shipping Package Segment
|
|
|
DB |
|
[{
|
--- SPECIMEN_IN_PACKAGE begin
|
|
|
|
|
SPM
|
Specimen Information
|
|
13.4.3
|
DB |
|
[{
|
--- SPECIMEN_CONTAINER_IN_PACKAGE begin
|
|
|
|
|
SAC
|
Container Information
|
|
7.4.3
|
DB |
|
}]
|
--- SPECIMEN_CONTAINER_IN_PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_IN_PACKAGE end
|
|
|
|
|
}
|
--- PACKAGE end
|
|
|
|
|
}]
|
--- SPECIMEN_SHIPMENT end
|
|
|
|
|
]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.14 OMI - Imaging Order Message (Event O23)
This message is used in communication between the information systems involved in the fulfillment of the request directed to the imaging department, such as a Radiology Information System (RIS) and a Picture Archiving and Communication System (PACS). For the purpose of the following discussion these systems will be identified as Imaging Department Information Systems (IDIS). Information contained in the Imaging Procedure Control (IPC) segment allows multiple IDIS to share the context of Imaging Studies (collections of images acquired, processed, stored, and interpreted) in Image Management tasks.
The order for the imaging service is communicated between the Order Placer (such as an Order Entry system) and the Order Filler (such as an RIS). In the imaging department environment, the Order Filler also identifies the set of procedures (studies) and sub-procedures (procedure steps) that have to be performed in the process of fulfilling the order. Each sub-procedure is performed using a single device (station). The Order Filler identifies the type of device and either a specific device or group of devices (for example, by geographic location) one of which is to be used in performing the procedure step. Thus, the system performs an aspect of workflow management in the department.
Another information system in the department may be managing storage and distribution of the images within the department as well as providing them to the enterprise. This system will have to operate within the same context as the system managing the workflow. This context includes identifiers, content of the order, and details of procedures and procedure steps that have to be performed to fulfill that particular order.
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
It is expected that the OMI message will typically be used in communication between IDIS as depicted in figure 4-1.
Figure 4-1 - Use of OMI message
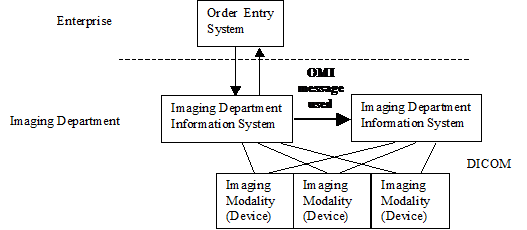
OMI^O23^OMI_O23: Imaging Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
OBR
|
Observation
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data
|
|
11
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
{
IPC
}
|
Imaging Procedure Control
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.4.15 ORI - Imaging Order Response Message to Any OMI (Event O24)
The function of this message is to respond to an OMI message. An ORI message is the application acknowledgment to an OMI message. See Chapter 2 for a description of the acknowledgment paradigm.
ORI^O24^ORI_O24: Imaging Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
OBR
|
Observation
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
{
IPC
}
|
Imaging Procedure Control
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.16 OPL - Population/Location-Based Laboratory Order Message (Event O37)
This message supports the use-case for submission of field level specimen and order data to diagnostic laboratories
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP"."
OPL^O37^OPL_O37: Population/Location-Based Laboratory Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for header)
|
|
2
|
DB |
|
{
PRT
}
|
Participation
|
|
7
|
DB |
|
[
|
--- GUARANTOR begin
|
|
|
|
|
GT1
|
Guarantor
|
|
6
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Guarantor)
|
|
2
|
DB |
|
]
|
--- GUARANTOR end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
{
NK1
}
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
|
---PATIENT begin
|
|
|
|
|
PID
|
Patient
|
|
3
|
DB |
|
[
PD1
]
|
Patient Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- OBSERVATIONS_ON_PATIENT begin
|
|
|
|
|
OBX
|
Observations on the Patient
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observations on Patient)
|
|
7
|
DB |
|
}]
|
--- OBSERVATIONS_ON_PATIENT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[{
|
--- CONTAINER begin
|
|
|
|
|
SAC
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- CONTAINER_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result related to container
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Containter Observation)
|
|
7
|
DB |
|
}]
|
--- CONTAINER_OBSERVATION end
|
|
|
|
|
}]
|
--- CONTAINER end
|
|
|
|
|
{
|
--- OBSERVATION REQUEST begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
TCD
]
|
Test Code Details
|
|
13
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- ORDER_RELATED_OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result Related to Order
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Related Observation)
|
|
7
|
DB |
|
}]
|
--- ORDER_RELATED_OBSERVATION end
|
|
|
|
|
}
|
--- OBSERVATION REQUEST end
|
|
|
|
|
}
|
--- SPECIMEN end
|
|
|
|
|
[
SGH
]
|
Segment Group Header
|
|
2
|
DB |
|
[
|
--- PRIOR_RESULT begin
|
|
|
|
|
{
NK1
}
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
|
--- PATIENT PRIOR begin
|
|
|
|
|
PID
|
Patient
|
|
3
|
DB |
|
[
PD1
]
|
Patient Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Information
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
AL1
]
|
Patient Allergy Information
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
ORC
]
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior)
|
|
7
|
DB |
|
[
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Relationship
|
|
4
|
DB |
|
]
|
--- TIMING end
|
|
|
|
|
{
|
--- OBSERVATION_RESULT_GROUP begin
|
|
|
|
|
OBX
|
Observation/Result for prior order
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation/Result)
|
|
7
|
DB |
|
}
|
--- OBSERVATION_RESULT_GROUP end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
SGT
]
|
Segment Group Trailer
|
|
2
|
DB |
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
This structure represents the way that most orders to veterinary laboratories occur. There is a multi-tier hierarchy in which a single individual (usually a veterinarian or an owner of a production facility) submits one or more specimen samples from one or more animals or non-living entities, such as environmental specimens or feed, etc. There are often many interested participants referenced for each set of orders, which explains the need for the repeating PRT segment. These include individuals such as the government official that is responsible for monitoring the testing of an animal or animal group, the parent organization, etc. This grouped submission of specimens from multiple animal "patients" requires that orders pertaining to animal and non-animal specimens be accommodated. The primary structure of concern is the following:
{[PID]
[{ARV}]
{SPM
{ORC
OBR}
}
}
This allows for multiple specimens or animal or non-animal origin to have multiple requests associated with them. This is the usual process in field level sample collection from populations or environments.
4.4.17 OPR - Population/Location-Based Laboratory Order Acknowledgment Message (Event O38)
The function of this message is to respond to an OPL message. An OPR message is the application acknowledgment to an OPL message. See Chapter 2 for a description of the acknowledgment paradigm.
Note: Based upon general message/acknowledgment patterns, it would be expected that this message type would be ORP. However, when this message type was introduced, ORP was already in use as Pharmacy/Treatment Order Acknowledgment.
OPR^O38^OPR_O38: Population/Location-Based Laboratory Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
{
NK1
}
|
Next of Kin
|
|
3
|
DB |
|
[
PID
]
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- SPECIMEN begin
|
|
|
|
|
SPM
|
Specimen
|
|
7
|
DB |
|
[{
|
--- SPECIMEN_OBSERVATION begin
|
|
|
|
|
OBX
|
Observations related to specimen
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Specimen Observation)
|
|
7
|
DB |
|
}]
|
--- SPECIMEN_OBSERVATION end
|
|
|
|
|
[
{
SAC
}
]
|
Specimen Container
|
|
13
|
DB |
|
[{
|
--- OBSERVATION_REQUEST begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Observation Request
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation Request)
|
|
7
|
DB |
|
}]
|
--- OBSERVATION_REQUEST end
|
|
|
|
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
}]
|
--- SPECIMEN end
|
|
|
|
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.4.18 OSU - Order Status Update (Event O41)
This message is used to create simple order status updates for any type of order where the ORC is sufficient to communicate the order identifier and no other data changes. This is particularly necessary when status updates are not part of order acknowledgement messages, e.g., a status message occurs 2 days later.
OSU^O41^OSU_O41: Order Status Update Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
PID
]
|
Patient Identification
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
{
|
--- ORDER STATUS begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
}
|
--- ORDER STATUS end
|
|
|
|
4.4.19 OMQ - General Order Message with Document Payload (Event O42)
The purpose of this message is to enable communication of orders using a CDA document type to convey the content of the order (e.g., prescription, lab tests, etc.) while the message infrastructure enables appropriate state management.
It should be noted that, unless orders are communicated at the granular, fully decomposed test/medication/procedure/etc. level, state management can only happen at the group level, i.e., equal to all elements in the document. It also should be noted that identification of individual elements can only be achieved if the CDA document contains appropriate identification while the order numbers in ORC effectively act as a group number.
Once the order manager determines to initiate a new order using this message, then all subsequent state management messages must continue at the document level, forgoing detailed level state management.
When one wants to convey with the detailed order message a supporting document, such as a CDA, one can transmit that document using the OBX associated with the ORC/OBR(s) using OBX-11 = "O" Order Detail Description Only, using either OBX-2 = "ED" or "RP".
OMQ^O42^OMQ_O42: General Order Message with Document Payload
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
NK1
}
]
|
Next of Kin/Associated Parties
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit- Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation (for Common Order)
|
|
7
|
DB |
|
OBX
|
Observation containing document
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
TXA
|
Transcription Document Header
|
|
9
|
DB |
|
[
CTD
]
|
Contact Data
|
|
11
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Observation)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[{
|
--- PRIOR_RESULT begin
|
|
|
|
|
[
|
--- PATIENT_PRIOR begin
|
|
|
|
|
PID
|
Patient Identification - previous result
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Prior)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT_PRIOR end
|
|
|
|
|
[
|
--- PATIENT_VISIT_PRIOR begin
|
|
|
|
|
PV1
|
Patient Visit - previous result
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit Add. Info - previous result
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit Prior)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT_PRIOR end
|
|
|
|
|
[
{
AL1
}
]
|
Allergy Information - previous result
|
|
3
|
DB |
|
{
|
--- ORDER_PRIOR begin
|
|
|
|
|
ORC
|
Common Order - previous result
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
OBR
|
Order Detail - previous result
|
|
4
|
DB |
|
[{
|
--- TIMING_PRIOR begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_PRIOR end
|
|
|
|
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Order Prior) - previous result
|
|
7
|
DB |
|
[
CTD
]
|
Contact Data - previous result
|
|
10
|
DB |
|
{
|
--- OBSERVATION_PRIOR begin
|
|
|
|
|
OBX
|
Observation/Result - previous result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation (for Oservation Prior)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments - previous result
|
|
2
|
DB |
|
}
|
--- OBSERVATION_PRIOR end
|
|
|
|
|
}
|
--- ORDER_PRIOR end
|
|
|
|
|
}]
|
--- PRIOR_RESULT end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.4.20 ORX - General Order Message with Document Payload Acknowledgement Message (Event O43)
The function of this message is to respond to an OMQ message. An ORX message is the application acknowledgment to an OMQ message. See Chapter 2 for a description of the acknowledgment paradigm.
In ORX the PID and ORC segments are optional, particularly in case of an error response. However, ORC segments are always required in ORD when the OBR is present. For example, a response ORD might include only the MSH and MSA.
The function (e.g., cancel, new order) of both OMQ and ORX messages is determined by the value in ORC-1-order control. (See the table of order control values for a complete list.)
ORX^O43^ORX_O43: General Order Message with Document Payload Acknowledgement Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
TXA
|
|
|
|
|
|
[
{
CTI
}
]
|
Clinical Trial Identification
|
|
7
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.5 GENERAL SEGMENTS
The following segments (ORC and BLG) are common to many order messages.
4.5.1 ORC - Common Order Segment
The Common Order segment (ORC) is used to transmit fields that are common to all orders (all types of services that are requested).
There is some overlap between fields of the ORC and those in the order detail segments. These are described in the succeeding sections.
HL7 Attribute Table - ORC - Common Order
ORC use notes
-
placer order groups
The Standard supports a mechanism to collect several orders together in a group. Most often this is used to represent an "ordering session" for a single patient. A common use case is the grouping of laboratory batteries and tests ordered together by a physician for a patient with a common diagnostic goal (e.g. preoperative blood testing, diabetes follow-up, ...).
An order group is a list of orders (ORCs) associated with an ORC-4-placer group number. A group is established when the placer supplies a placer group number with the original order or when the filler accessions the order group and supplies a filler group number with the received order. The order group may be identified by the placer or by the filler or by both applications. The order group consists of all the ORCs and order detail segments that have the same placer group number, or using the assign number/number assigned mechanism. Orders can be removed from the group using cancel, or added using the replacement or parent-child mechanisms. New orders cannot otherwise be added to the group.
-
duplicate fields
The ORC is intended to uniformly define the fields that are common to all orders (i.e., requested services). Some ORC fields are duplicated in some order detail segments (e.g., OBR, RXO). For example, ORC-2-placer order number has the same meaning and purpose as OBR-2-placer order number field. This promotes upward compatibility with past versions and ASTM.
The rule for using these fields is that the value must appear in the order detail segment if it does not appear in the ORC. However, it is recommended to transmit the field value in both places to avoid confusion.
-
parent/child - cancel, hold, discontinue
During transmission of a request to cancel, hold, or discontinue a parent order, the request is intended to apply recursively to the parent order and all associated child orders.
For example:
-
An EKG application receives an order for three EKGs on successive mornings.
-
The EKG application creates three child orders, one for each requested EKG..
-
The first daily EKG has already been performed when a request is received to cancel the original parent order. (The parent is beyond the point of cancellation.)
-
The remaining, unperformed, children are canceled as a result of the request.
4.5.1.0 ORC field definitions
4.5.1.1 ORC-1 Order Control (ID) 00215
Definition: Determines the function of the order segment. Refer to HL7 Table 0119 - Order Control Codes in Chapter 2C, Code Tables, for valid entries. Depending on the message, the action of the control code may refer to an order or an individual service. For example, the code CA in an OMP message cancels the order. The same code in an RDS message, cancels the dispense. Very detailed explanatory notes are given at the end of this section.
This field may be considered the "trigger event" identifier for orders. The codes fall roughly into the following three categories:
-
event request - Codes like "NW" (new order) and "CA" (cancel order request) are used to initiate an event .
-
event acknowledgment - Codes like "OK" (order accepted) and "CR" (canceled as requested) are used to reply to the event request .
-
event notification - Codes like "OC" (order canceled) and "OD" (order discontinued) are used to notify other applications that an event has occurred. No application reply is necessary.
Event request codes are intended to initiate an event. Event acknowledgment codes are intended to reply to an application that requested an event. Event notification codes are intended to notify another application that, e.g., the filler has performed some action on an order that the other application, e.g., the placer, needs to know.
Fillers, placers, and other applications can use event requests, event acknowledgments, and event - notification-type trigger events interchangeably. However, certain order control codes can originate only from the filler (e.g., CR) and others can only originate from the placer (e.g., CA).
Refer HL7 Table 0119 - Order Control Codes in Chapter 2C, Code Tables.
4.5.1.2 ORC-2 Placer Order Number (EI) 00216
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is the placer application's order number.
This field is a case of the Entity Identifier data type (See Section 2.A.28, "EI - Entity Identifier"). The first component is a string that identifies an individual order (i.e., ORC segment and associated order detail segment). It is assigned by the placer (ordering application). It identifies an order uniquely among all orders from a particular ordering application. The second through fourth components contain the application ID of the placing application in the same form as the HD data type (Section 2.A.36, "HD - Hierarchic designator"). The second component, namespace ID, is a user-defined coded value that will be uniquely associated with an application. A limit of six (6) characters is suggested but not required. A given institution or group of intercommunicating institutions should establish a unique list of applications that may be potential placers and fillers and assign unique application IDs. The components are separated by component delimiters.
There are three situations in which the true placer is somewhat arbitrary (and thus not unique):
-
in ORC-1-order control value of RO, following an RU replacement;
-
in ORC-1-order control value of CH (child orders); and
-
in ORC-1-order control value of SN (send number).
See the Table Notes under ORC-1-order control for the details of how the ORC-2-placer order number is assigned in these cases.
The application ID list becomes one of the institution's master dictionary lists that is documented in Chapter 8. Since third-party applications (those other than the placer and filler of an order) can send and receive ORM and ORR messages, the placer application ID in this field may not be the same as any sending and receiving application on the network (as identified in the MSH segment).
The conditions which make this field required are divided into two main issues. The data in ORC-2 and OBR-2 are logically the same thing: a placer id. The data in ORC-3 and OBR-3 are logically the same thing: the filler id.
From that perspective each message must have either a placer or a filler id with an exception for the case of a "Send Number" control code since its purpose is to request a placer id.
If both ORC and OBR are present in a message, then only one of the Segments must contain the value(s). Note that if both ORC-2 and OBR-2 are valued then they must be valued the same; as well, if both ORC-3 and OBR-3 are valued, then they must be valued the same. The sending system can include both the filler and the placer number in both the ORC and OBR segments as long as the data is the same between the two segments.
It is recommended that the initiating system should provide a unique number for the placer order number when a new order is placed or a unique number for the filler order number when an unsolicited result is initially communicated.
These rules apply to the few other fields that are present in both ORC and OBR for upward compatibility (e.g., quantity/timing, parent numbers, ordering provider, and ordering call back numbers).
4.5.1.3 ORC-3 Filler Order Number (EI) 00217
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is the order number associated with the filling application. It is a case of the Entity Identifier data type (Section 2.A.28). Its first component is a string that identifies an order detail segment (i.e., ORC segment and associated order detail segment). It is assigned by the order filler (receiving) application. This string must uniquely identify the order (as specified in the order detail segment) from other orders in a particular filling application (e.g., clinical laboratory). This uniqueness must persist over time.
The second through fourth components contain the filler application ID, in the form of the HD data type (see Section 2.A.36, "HD - hierarchic designator"). The second component is a user-defined coded value that uniquely defines the application from other applications on the network. A limit of six (6) characters is suggested but not required. The second component of the filler order number always identifies the actual filler of an order.
A given institution or group of intercommunicating institutions should establish a list of applications that may be potential placers and fillers of orders and assign each a unique application ID. The application ID list becomes one of the institution's master dictionary lists that is documented in Chapter 8. Since third- party applications (those other than the placer and filler of an order) can send and receive ORM and ORR messages, the filler application ID in this field may not be the same as any sending and receiving application on the network (as identified in the MSH segment).
The conditions which make this field required are divided into two main issues. The data in ORC-2 and OBR-2 are logically the same thing: a placer id. The data in ORC-3 and OBR-3 are logically the same thing: the filler id.
From that perspective each message must have either a placer or a filler id with an exception for the case of a "Send Number" control code since its purpose is to request a placer id.
If both ORC and OBR are present in a message, then only one of the Segments must contain the value(s). Note that if both ORC-2 and OBR-2 are valued, then they must be valued the same; as well, if both ORC-3 and OBR-3 are valued, then they must be valued the same. The sending system can include both the filler and the placer number in both the ORC and OBR segments as long as the data is the same between the two segments. It is recommended that the initiating system should provide a unique number for the placer order number when a new order is placed or a unique number for the filler order number when an unsolicited result is initially communicated.
The filler order number (OBR-3 or ORC-3) also uniquely identifies an order and its associated observations. For example, suppose that an institution collects observations from several ancillary applications into a common database and this common database is queried by yet another application for observations. In this case, the filler order number and placer order number transmitted by the common database application would be that of the original filler and placer, respectively, rather than a new one assigned by the common database application.
Similarly, if a third-party application, not the filler or placer, of an order were authorized to modify the status of an order (say, cancel it), the third-party application would send the filler an ORM message containing an ORC segment with ORC-1-order control equal to "CA" and containing the original placer order number and filler order number, rather than assign either itself.
4.5.1.4 ORC-4 Placer Group Number (EIP) 00218
Components: <Placer Assigned Identifier (EI)> ^ <Filler Assigned Identifier (EI)>
Subcomponents for Placer Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Filler Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains a unique identifier for the Placer Group as referenced by the Placer application, the Filler application, or both. A Placer Group is a set of orders grouped together by the placer application, and subsequently identified by the placer application and/or by the filler application.
Within each of the two Subcomponents, the first component is a string that uniquely identifies all order groups from the given (placer or filler) application. A limit of fifteen (15) characters is suggested but not required.
The second through fourth components constitute a (placer or filler) application ID identical to the analogous components of ORC-2-placer order number or ORC-3-filler order number . Order groups and how to use them are described in detail in Section 4.5.1, "ORC - Common Order Segment."
4.5.1.5 ORC-5 Order Status (ID) 00219
Definition: This field specifies the status of an order. Refer to HL7 Table 0038 - Order status in Chapter 2C, Code Tables, for valid entries. The purpose of this field is to report the status of an order either upon request (solicited), or when the status changes (unsolicited). It does not initiate action. It is assumed that the order status always reflects the status as it is known to the sending application at the time that the message is sent. Only the filler can originate the value of this field.
Although HL7 Table 0038 - Order status contains many of the same values contained in HL7 Table 0119 - Order control codes and their meaning, its purpose is different. Order status may typically be used in a message with an ORC-1-order control value of SR or SC to report the status of the order on request or to any interested party at any time.
4.5.1.6 ORC-6 Response Flag (ID) 00220
Definition: This field allows the placer (sending) application to determine the amount of information to be returned from the filler. Sometimes the requested level of response may not be possible immediately, but when it is possible, the filler (receiving) application must send the information. When the field is null, D is the default value of the field. Refer to HL7 Table 0121 - Response flag in Chapter 2C, Code Tables, for valid entries.
4.5.1.7 ORC-7 Quantity/Timing
Attention: The ORC-7 field was retained for backward compatibilty only as of v 2.5 and the detail was withdrawn and removed from the standard as of v 2.7. The reader is referred to the TQ1 and TQ2 segments described in sections 4.5.4 and 4.5.5, respectively.
4.5.1.8 ORC-8 Parent Order (EIP) 00222
Components: <Placer Assigned Identifier (EI)> ^ <Filler Assigned Identifier (EI)>
Subcomponents for Placer Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Filler Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field relates a child order to its parent order when a parent child order relationship exists. The parent child order mechanism is described in HL7 Table 0119 under order control code PA. This field uniquely identifies the parent order; no other information is required to link the child order with its parent order.
The first component has the same format as ORC-2-Placer Order Number (Section â??4.5.3.2, "Placer Order Number (EI) 00216"). The second component has the same format as ORC-3-Filler Order Number (Section â??4.5.3.3, "Filler Order Number (EI) 00217"). The components of the placer order number and the filler order number are transmitted in sub-components of the two components of this field.
Note that ORC-8 - Parent Order is equivalent to OBR-54 - Parent Order, but neither one is the same as OBR-29 - Result Observation Identifier.
Condition: Where the message has matching ORC/OBR pairs, ORC-8 and OBR-54 Must carry the same value.
4.5.1.9 ORC-9 Date/Time of Transaction (DTM) 00223
Definition: This field contains the date and time of the event that initiated the current transaction as reflected in ORC-1 Order Control Code. This field is not equivalent to MSH-7 Date and Time of Message which reflects the date/time of the physical message.
4.5.1.10 ORC-10 Entered By (XCN) 00224
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the identity of the person who actually keyed the request into the application. Note that this refers to the current transaction as reflected in ORC-1 Order Control Code. It provides an audit trail in case the request is entered incorrectly and the ancillary department needs to clarify the request. By local agreement, either the ID number or name component may be omitted. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.1.11 ORC-11 Verified By (XCN) 00225
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the identity of the person who verified the accuracy of the entered request. Note that this refers to the current transaction as reflected in ORC-1 Order Control Code. It is used in cases where the request is entered by a technician and needs to be verified by a higher authority (e.g., a nurse). By local agreement, either the ID number or name component may be omitted. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.1.12 ORC-12 Ordering Provider (XCN) 00226
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the identity of the person who is responsible for creating the request (i.e., ordering physician).
ORC-12-ordering provider is the same as OBR-16-ordering provider. If the ordering provider is not present in the ORC, it must be present in the associated OBR. (This rule is the same for other identical fields in the ORC and OBR and promotes upward and ASTM compatibility.) This is particularly important when results are transmitted in an ORU message. In this case, the ORC is not required and the identifying filler order number must be present in the OBR segments. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.1.13 ORC-13 Enterer's Location (PL) 00227
Components: <Point of Care (HD)> ^ <Room (HD)> ^ <Bed (HD)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Person Location Type (IS)> ^ <Building (HD)> ^ <Floor (HD)> ^ <Location Description (ST)> ^ <Comprehensive Location Identifier (EI)> ^ <Assigning Authority for Location (HD)>
Subcomponents for Point of Care (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Room (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Bed (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Building (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Floor (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Comprehensive Location Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Authority for Location (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field specifies the location (e.g., nurse station, ancillary service location, clinic, floor) where the person who entered the request was physically located when the order was entered. Note that this refers to the current transaction as reflected in ORC-1 Order Control Code. Only those subcomponents relevant to enterer's location should be valued (commonly, nursing unit; facility; building; floor). The person who entered the request is defined in ORC-10-entered by.
4.5.1.14 ORC-14 Call Back Phone Number (XTN) 00228
Components: <WITHDRAWN Constituent> ^ <Telecommunication Use Code (ID)> ^ <Telecommunication Equipment Type (ID)> ^ <Communication Address (ST)> ^ <Country Code (SNM)> ^ <Area/City Code (SNM)> ^ <Local Number (SNM)> ^ <Extension (SNM)> ^ <Any Text (ST)> ^ <Extension Prefix (ST)> ^ <Speed Dial Code (ST)> ^ <Unformatted Telephone number (ST)> ^ <Effective Start Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Protection Code (CWE)> ^ <Shared Telecommunication Identifier (EI)> ^ <Preference Order (NM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Shared Telecommunication Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the telephone number to call for clarification of a request or other information regarding the order. ORC-14-call back phone number is the same as OBR-17-order callback phone number.
4.5.1.15 ORC-15 Order Effective Date/Time (DTM) 00229
Definition: This field contains the date/time that the changes to the request took effect or are supposed to take effect.
If ORC-9-date/time of transaction is after or equal to ORC-15-order effective date/time, the data values in the ORC and its subordinate segments took effect on the order effective date/time.
If ORC-9-date/time of transaction is before the time specified in ORC-15-order effective date/time, the data values in ORC and its subordinate segments are planned to take effect on the order effective date/time.
If ORC-15-order effective date/time is left blank, its value is assumed to be equal to that specified in ORC-9-date/time of transaction or MSH-7-date/time of message if the transaction date/time is blank.
In the case where the time specified in ORC-15-order effective date/time (for the order control code event in the same ORC segment) is different from the corresponding date/time in TQ1 timing/quantity, the time specified in ORC-15-order effective date/time takes precedence. Thus if the ORC event is a discontinue request to the filler for a continuing order, and the order-effective date/time is prior to the end date/time of TQ1 timing/quantity, the order effective date/time should take precedence. If the order identified in the ORC has children, the children which have not started should be canceled; if there is a child in process, it should be discontinued; if a child has progressed beyond the point where it can be discontinued, its status is unaffected.
4.5.1.16 ORC-16 Order Control Code Reason (CWE) 00230
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the explanation (either in coded or text form) of the reason for the order event described by the order control code (HL7 Table 0119 - Order control codes). Whereas an NTE after the order-specific segment (e.g., RXO, ORO, OBR) would provide a comment for that specific segment, the purpose of the order control code reason is only to expand on the reason for the order event.
ORC-16-order control code reason is typically not valued when ORC-1-order control is NW, although it could be. In the case of a canceled order, for example, this field is commonly used to explain the cancellation. A Pharmacy system that canceled a drug order from a physician because of a well-documented allergy would likely report the fact of the allergy in this field.
If it canceled the order because of a drug interaction this field might contain at least the names (and codes, if needed) of the interacting substances, the text describing the interaction, and the level of severity of the interaction.
4.5.1.17 ORC-17 Entering Organization (CWE) 00231
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field identifies the organization that the enterer belonged to at the time he/she enters/maintains the order, such as medical group or department. The person who entered the request is defined in ORC-10-entered by.
4.5.1.18 ORC-18 Entering Device (CWE) 00232
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field identifies the physical device (terminal, PC) used to enter the order.
4.5.1.19 ORC-19 Action By (XCN) 00233
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the identity of the person who initiated the event represented by the corresponding order control code. For example, if the order control code is CA (cancel order request), this field represents the person who requested the order cancellation. This person is typically a care provider but may not always be the same as ORC-12 ordering provider. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.1.20 ORC-20 Advanced Beneficiary Notice Code (CWE) 01310
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the status of the patient's or the patient's representative's consent for responsibility to pay for potentially uninsured services. This element is introduced to satisfy CMS Medical Necessity requirements for outpatient services. This element indicates (a) whether the associated diagnosis codes for the service are subject to medical necessity procedures, (b) whether, for this type of service, the patient has been informed that they may be responsible for payment for the service, and (c) whether the patient agrees to be billed for this service. The values for this field are drawn from User-Defined Table 0339 - Advanced Beneficiary Notice Code in Chapter 2C, Code Tables.
4.5.1.21 ORC-21 Ordering Facility Name (XON) 01311
Components: <Organization Name (ST)> ^ <Organization Name Type Code (CWE)> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Organization Identifier (ST)>
Subcomponents for Organization Name Type Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the name of the facility placing the order.
4.5.1.22 ORC-22 Ordering Facility Address (XAD) 01312
Components: <Street Address (SAD)> ^ <Other Designation (ST)> ^ <City (ST)> ^ <State or Province (ST)> ^ <Zip or Postal Code (ST)> ^ <Country (ID)> ^ <Address Type (ID)> ^ <Other Geographic Designation (ST)> ^ <County/Parish Code (CWE)> ^ <Census Tract (CWE)> ^ <Address Representation Code (ID)> ^ <WITHDRAWN Constituent> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Temporary Indicator (ID)> ^ <Bad Address Indicator (ID)> ^ <Address Usage (ID)> ^ <Addressee (ST)> ^ <Comment (ST)> ^ <Preference Order (NM)> ^ <Protection Code (CWE)> ^ <Address Identifier (EI)>
Subcomponents for Street Address (SAD): <Street or Mailing Address (ST)> & <Street Name (ST)> & <Dwelling Number (ST)>
Subcomponents for County/Parish Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Census Tract (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Address Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the address of the facility placing the order.
4.5.1.23 ORC-23 Ordering Facility Phone Number (XTN) 01313
Components: <WITHDRAWN Constituent> ^ <Telecommunication Use Code (ID)> ^ <Telecommunication Equipment Type (ID)> ^ <Communication Address (ST)> ^ <Country Code (SNM)> ^ <Area/City Code (SNM)> ^ <Local Number (SNM)> ^ <Extension (SNM)> ^ <Any Text (ST)> ^ <Extension Prefix (ST)> ^ <Speed Dial Code (ST)> ^ <Unformatted Telephone number (ST)> ^ <Effective Start Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Protection Code (CWE)> ^ <Shared Telecommunication Identifier (EI)> ^ <Preference Order (NM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Shared Telecommunication Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the telephone number of the facility placing the order.
4.5.1.24 ORC-24 Ordering Provider Address (XAD) 01314
Components: <Street Address (SAD)> ^ <Other Designation (ST)> ^ <City (ST)> ^ <State or Province (ST)> ^ <Zip or Postal Code (ST)> ^ <Country (ID)> ^ <Address Type (ID)> ^ <Other Geographic Designation (ST)> ^ <County/Parish Code (CWE)> ^ <Census Tract (CWE)> ^ <Address Representation Code (ID)> ^ <WITHDRAWN Constituent> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Temporary Indicator (ID)> ^ <Bad Address Indicator (ID)> ^ <Address Usage (ID)> ^ <Addressee (ST)> ^ <Comment (ST)> ^ <Preference Order (NM)> ^ <Protection Code (CWE)> ^ <Address Identifier (EI)>
Subcomponents for Street Address (SAD): <Street or Mailing Address (ST)> & <Street Name (ST)> & <Dwelling Number (ST)>
Subcomponents for County/Parish Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Census Tract (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Address Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
This field contains the address of the care provider requesting the order. If the address referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.1.25 ORC-25 Order Status Modifier (CWE) 01473
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is a modifier or refiner of the ORC-5-Order status field. This field may be used to provide additional levels of specificity or additional information for the defined order status codes. Unlike the Order Status field, which is controlled by an HL7 defined table, this field is a CE data type allowing applications to support an unlimited library of Order Status Modifier codes.
Usage Rule: This field may only be populated if the ORC-5-Order Status field is valued.
Examples: An LIS processing an order with an order status of IP may send an update using the order status modifier to indicate the progress of the order through the laboratory or to indicate that the order has been sent to an external laboratory. Another example using the non-medical orders would be a case in which a phone has been ordered delivered to a patient's room but has been disconnected temporarily. The ORC-5-Order status indicates IP and the ORC-25-Order status modifier would indicate a disconnected status. A third example involves pharmacy dispenses. It is sometimes not enough to know that a prescription is being dispensed. The ORC-25-Order status modifier would indicate if a label had been printed, the prescription filled, or the prescription sold.
4.5.1.26 ORC-26 Advanced Beneficiary Notice Override Reason (CWE) 01641
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the reason why the patient did not sign an Advanced Beneficiary Notice. The reason may be coded or it may be a free text entry. Refer to HL7 Table 0552 - Advanced beneficiary notice override reason in Chapter 2C, Code Tables.
Condition: This field is required if the value of ORC-20 Advanced Beneficiary Notice Code indicates that the notice was not signed. For example, additional qualifying or explanatory information would be justified if ORC-20 was populated with the values "3" or "4" in User-defined Table 0339 - Advanced Beneficiary Notice Code, or similar values in related external code tables.
4.5.1.27 ORC-27 Filler's Expected Availability Date/Time (DTM) 01642
Definition: This field specifies the date/time the filler expects the services to be available. For example when a prescription is ready for pickup or when a supply will be sent or picked up, or for when a laboratory result is expected to be available.
4.5.1.28 ORC-28 Confidentiality Code (CWE) 00615
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains information about the level of security and/or sensitivity surrounding the order (e.g., highly sensitive, not sensitive, sensitive, etc.). Refer to HL7 Table 0177 - Confidentiality Code in Chapter 2C, Code Tables, for allowed values. The specific treatment of data with a particular confidentiality level is subject to site-specific negotiation.
4.5.1.29 ORC-29 Order Type (CWE) 01643
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates whether the order is to be executed in an inpatient setting or an outpatient setting. If this field is not valued, the system default is assumed. Refer to HL7 Table 0482 - Order Type in Chapter 2C, Code Tables, for suggested values.
Examples: Before discharge an order is placed for follow-up physical therapy, or to pick up a prescription at a community pharmacy. The patient is an inpatient according to PV1, but the order is an outpatient order.
4.5.1.30 ORC-30 Enterer Authorization Mode (CNE) 01644
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the form of authorization a recorder had from the responsible practitioner to create or change an order. Refer to HL7 Table 0483 - Authorization Mode in Chapter 2C, Code Tables, for suggested values.
4.5.1.31 ORC-31 Parent Universal Service Identifier (CWE) 02287
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Retained for backward compatibility only as of v 2.7. This field contains the identifier code for the parent order which caused this reflex observation/test/battery to be performed. This can be based on local and/or "universal" codes. We recommend the "universal" service identifier.
ORC-31 - parent universal service identifier is the same as OBR-50 - parent universal service identifier. If both fields are valued, they must contain the same value.
4.5.1.32 ORC-32 Advanced Beneficiary Notice Date (DT) 02301
Definition: This field contains the date the patient gave consent to pay for potentially uninsured services or the date that the Advanced Beneficiary Notice Code (ORC-20) was collected.
4.5.1.33 ORC-33 Alternate Placer Order Number (CX) 03300
Components: <ID Number (ST)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Effective Date (DT)> ^ <Expiration Date (DT)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field enables a shorter number to be communicated that is unique within other identifiers.
4.5.1.34 ORC-34 Order Workflow Profile (EI) 03387
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
The Order Workflow Profile references/represents the information necessary to define the workflow variant when that is not fully described through the use of ORC-1 Order Control and MSH-21 Message Profile. This enables contributing systems to apply locally agreed to rules. See User-defined Table 0934 - Order Workflow Profile for a list of suggested values.
4.5.2 BLG - Billing Segment
The BLG segment is used to provide billing information, on the ordered service, to the filling application.
HL7 Attribute Table - BLG - Billing
4.5.2.0 BLG field definitions
4.5.2.1 BLG-1 When to charge (CCD) 00234
Components: <Invocation Event (ID)> ^ <Date/time (DTM)>
Definition: This field specifies when to charge for the ordered service. Refer to HL7 Table 0100 - Invocation event in Chapter 2C, Code Tables, for valid values.
4.5.2.2 BLG-2 Charge type (ID) 00235
Definition: This field identifies someone or something other than the patient to be billed for this service. It is used in conjunction with BLG-3-account ID. Refer to HL7 Table 0122 - Charge Type in Chapter 2C, Code Tables, for valid values.
4.5.2.3 BLG-3 Account ID (CX) 00236
Components: <ID Number (ST)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Effective Date (DT)> ^ <Expiration Date (DT)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field identifies the account to be billed. It is used in conjunction with BLG-2-charge type. Refer to HL7 Table 0061 - Check digit scheme in Chapter 2C, Code Tables.
4.5.2.4 BLG-4 Charge type reason (CWE) 01645
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field explains the choice of and provides the clinical rationale for the selected charge type identified in BLG-2. Refer to User-defined Table 0475 - Charge Type Reason in Chapter 2C, code Tables, for suggested values.
4.5.3 OBR - Observation Request Segment
General (taken from ASTM E1238)
The Observation Request (OBR) segment is used to transmit information specific to an order for a diagnostic study or observation, physical exam, or assessment.
The Observation Request segment defines the attributes of a particular request for diagnostic services (e.g., laboratory, EKG) or clinical observations (e.g., vital signs or physical exam). When a placer requests a given set of observations, always include an order segment. For lab tests, the information in the order segment usually applies to a single specimen. However, there is not a one-to-one relationship between specimen and tests ordered. Different test batteries will usually require their own order segments even when they can be performed on a single specimen. In this case, the specimen information must be duplicated in each of the order segments that employ that specimen. For other diagnostic studies, e.g., chest X-ray, a separate order segment will usually be generated for each diagnostic study.
Though multiple observation batteries can be ordered on a single order segment, the observation filler shall generate a separate order segment for each battery that it processes independently, e.g., electrolyte, CBC, vital signs. When reporting the observations, the filling service shall copy the appropriate order (specimen) information from the original order segment into each of the new order segments so that a separate "order" segment is returned to the placer as a "header" for each separate battery of observations.
In the event that an ordered battery of observations cannot be performed, e.g., because of hemolysis on a blood sample, an order segment will be returned to the placer with OBR-25-result status equal to X (to indicate that the study was not performed). In this case, no observation segments will be transmitted.
When observations are successfully completed, the message returned to the placer will include the order segment (OBR) followed by observation (OBX) segments for each distinct observation generated by the order (see Chapter 7). The number of such observation segments will depend upon the number of individual measurements performed in the process.
OBX segments can be sent by the placer along with an order to provide the filling service with clinical data needed to interpret the results. (See Chapter 7 for OBX details.)
HL7 Attribute Table - OBR - Observation Request
4.5.3.0 OBR field definitions
The daggered (+) items in this segment are created by the filler, not the placer. They are valued by the filler as needed when the OBR segment is returned as part of a report.
The starred (*) fields are only relevant when an observation is associated with a specimen. These are completed by the placer when the placer obtains the specimen. They are completed by the filler when the filler obtains the specimen.
OBR-7-observation date/time and OBR-8-observation end date/time (flagged with #) are the physiologically relevant times. In the case of an observation on a specimen, they represent the start and end of the specimen collection. In the case of an observation obtained directly from a subject (e.g., BP, Chest X-ray), they represent the start and end time of the observation.
4.5.3.1 OBR-1 Set ID - OBR (SI) 00237
Definition: For the first order transmitted, the sequence number shall be 1; for the second order, it shall be 2; and so on.
4.5.3.2 OBR-2 Placer order number (EI) 00216
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is identical to ORC-2-Placer Order Number.
This field is a special case of the Entity Identifier data type (Chapter 2A, section 2.A.28). The first component is a string that identifies an individual order (i.e., ORC segment and associated order detail segment). A limit of fifteen (15) characters is suggested but not required. It is assigned by the placer (ordering application). An implementation is HL7 compliant when the number of characters for this field is increased to accommodate applications that require a greater number of characters for the Placer order number. It identifies an order uniquely among all orders from a particular ordering application. The second through fourth components contain the application ID of the placing application in the same form as the HD data type (section 2.A.36, "HD - Hierarchic designator"). The second component, namespace ID, is a user-defined coded value that will be uniquely associated with an application. A limit of six (6) characters is suggested but not required. A given institution or group of intercommunicating institutions should establish a unique list of applications that may be potential placers and fillers and assign unique application IDs. The components are separated by component delimiters.
See ORC-2-placer order number (section 4.5.1.2) for information on when this field must be valued.
A given institution or group of intercommunicating institutions should establish a list of applications that may be potential placers and fillers of orders and assign each a unique application ID. The application ID list becomes one of the institution's master dictionary lists that is documented in Chapter 8. Since third-party applications (those other than the placer and filler of an order) can send and receive ORM and ORR messages, the placer application ID in this field may not be the same as any sending and receiving application on the network (as identified in the MSH segment).
The conditions which make this field required are divided into two main issues. The data in ORC-2 and OBR-2 are logically the same thing: a placer id. The data in ORC-3 and OBR-3 are logically the same thing: the filler id.
From that perspective, each message must have either a placer or a filler id with an exception for the case of a "Send Number" control code since its purpose is to request a placer id.
If both ORC and OBR are present in a message, then only one of the Segments must contain the value(s). If both segments contain either ORC-2/OBR-2 or ORC-3/OBR-3, then each pair must be a matching pair. The sending system can include both the filler and the placer number in both the ORC and OBR segments as long as the data is the same between the two segments.
It is recommended that the initiating system should provide a unique number when a new order or unsolicited result is initially communicated.
These rules apply to the few other fields that are present in both ORC and OBR for upward compatibility (e.g., quantity/timing, parent numbers, ordering provider, and ordering call back numbers).
4.5.3.3 OBR-3 Filler Order Number (EI) 00217
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is the order number associated with the filling application. This is a permanent identifier for an order and its associated observations. It is a special case of the Entity Identifier data type (see Chapter 2, section 2.A.28, "EI - entity identifier").
The first component is a string that identifies an individual order segment (i.e., ORC segment and associated order detail segment). It is assigned by the order filling (receiving) application. It identifies an order uniquely among all orders from a particular filling application (e.g., clinical laboratory). This uniqueness must persist over time.
The second through fourth components contain the filler application ID, in the form of the HD data type (see section 2.A.36, "HD - hierarchic designator"). The second component is a user-defined coded value that uniquely defines the application from other applications on the network. A limit of six (6) characters is suggested but not required. The second component of the filler order number always identifies the actual filler of an order.
See ORC-3-filler order number for information on when this field must be valued.
The conditions which make this field required are divided into two main issues. The data in ORC-2 and OBR-2 are logically the same thing: a placer id. The data in ORC-3 and OBR-3 are logically the same thing: the filler id.
From that perspective, each message must have either a placer or a filler id with an exception for the case of a "Send Number" control code since its purpose is to request a placer id.
If both ORC and OBR are present in a message, then only one of the Segments must contain the value(s). If both segments contain either ORC-2/OBR-2 or ORC-3/OBR-3, then each pair must be a matching pair. The sending system can include both the filler and the placer number in both the ORC and OBR segments as long as the data is the same between the two segments.
It is recommended that the initiating system should provide a unique number when a new order or unsolicited result is initially communicated.
The filler order number (OBR-3 or ORC-3) also uniquely identifies an order and its associated observations. For example, suppose that an institution collects observations from several ancillary applications into a common database and this common database is queried by yet another application for observations. In this case, the filler order number and placer order number transmitted by the common database application would be that of the original filler and placer, respectively, rather than a new one assigned by the common database application.
Similarly, if a third-party application, not the filler or placer, of an order were authorized to modify the status of an order (say, cancel it), the third-party application would send the filler an ORM message containing an ORC segment with ORC-1-order control equal to "CA" and containing the original placer order number and filler order number, rather than assign either itself.
4.5.3.4 OBR-4 Universal Service Identifier (CWE) 00238
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identifier code for the requested observation/test/battery. The identifier can come from either a local coding system or industry standards such as SNOMED and LOINC.
4.5.3.5 OBR-5 Priority
Attention: The OBR-5 element was retained for backward compatibilty only as of v 2.4 and the detail was withdrawn and removed from the standard as of v 2.7.
4.5.3.6 OBR-6 Requested Date/Time
Attention: The OBR-6 element was retained for backward compatibilty only as of v 2.4 and the detail was withdrawn and removed from the standard as of v 2.7.
4.5.3.7 OBR-7 Observation Date/Time (DTM) 00241
Definition: This field is the clinically relevant date/time of the observation. In the case of observations taken directly from a subject, it is the actual date and time the observation was obtained. In the case of a specimen-associated study, this field shall represent the date and time the specimen was collected or obtained. (This is a results-only field except when the placer or a third party has already drawn the specimen.) This field is conditionally required. When the OBR is transmitted as part of a report message, the field must be filled in. If it is transmitted as part of a request and a sample has been sent along as part of the request, this field must be filled in because this specimen time is the physiologically relevant date/time of the observation.
4.5.3.8 OBR-8 Observation End Date/Time (DTM) 00242
Definition: This field contains the end date and time of a study or timed specimen collection. If an observation takes place over a substantial period of time, it will indicate when the observation period ended. For observations made at a point in time, it will be null. This is a results field except when the placer or a party other than the filler has already drawn the specimen.
4.5.3.9 OBR-9 Collection Volume (CQ) 00243
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: For laboratory tests, the collection volume is the volume of a specimen. The default unit is ML. Specifically, units should be expressed in the ISO Standard unit abbreviations (ISO-2955, 1977). This is a results-only field except when the placer or a party has already drawn the specimen. (See Chapter 7 Section 7.4.2.6 for a full discussion regarding units.)
4.5.3.10 OBR-10 Collector Identifier (XCN) 00244
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. The reader is referred to the PRT segment described in Chapter 7.
When a specimen is required for the study, this field will identify the person, department, or facility that collected the specimen. Either name or ID code, or both, may be present. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.3.11 OBR-11 Specimen Action Code (ID) 00245
Definition: This field identifies the action to be taken with respect to the specimens that accompany or precede this order. The purpose of this field is to further qualify (when appropriate) the general action indicated by the order control code contained in the accompanying ORC segment. For example, when a new order (ORC - "NW") is sent to the lab, this field would be used to tell the lab whether or not to collect the specimen ("L" or "O"). Refer to HL7 Table 0065 - Specimen Action Code in Chapter 2C, Code Tables, for valid values.
4.5.3.12 OBR-12 Danger Code (CWE) 00246
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the code and/or text indicating any known or suspected patient or specimen hazards, e.g., patient with active tuberculosis or blood from a hepatitis patient. Either code and/or text may be absent. However, the code is always placed in the first component position and any free text in the second component. Thus, free text without a code must be preceded by a component delimiter.
4.5.3.13 OBR-13 Relevant Clinical Information (CWE) 00247
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains additional clinical information about the patient or specimen. This field is used to report the supporting and/or suspected diagnosis and clinical findings on requests for interpreted diagnostic studies where a simple text string or code is sufficient. This field could use all appropriate code sets including SNOMED to message Relevant Clinical Information. If more information is needed, such as date/time of the observation, who observed it, abnormal ranges, etc., or must be provided in further structured format, e.g., structured numeric with units of measure encoded, the Observation/Result group following the OBR should be used. Examples include reporting the amount of inspired carbon dioxide for blood gasses, the point in the menstrual cycle for cervical pap tests, and other conditions that influence test interpretations. Refer to HL7 Table 0916 - Relevant Clinical Information in Chapter 2C, Code Tables, for valid values.
4.5.3.14 OBR-14 Specimen Received Date/Time
Attention: The OBR-14 element was retained for backward compatibilty only as of v 2.5 and the detail was withdrawn and removed from the standard as of v 2.7. See SPM in Chapter 7.
4.5.3.15 OBR-15 Specimen Source
Attention: The OBR-15 element was retained for backward compatibilty only as of v 2.5 and the detail was withdrawn and removed from the standard as of v 2.7. See SPM in Chapter 7.
4.5.3.16 OBR-16 Ordering Provider (XCN) 00226
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 27. The reader is referred to the PRT segment described in Chapter 7.
This field identifies the provider who ordered the test. Either the ID code or the name, or both, may be present. This is the same as ORC-12-ordering provider. If the person referenced in this field is also referenced in PRT segment, they must contain the same information. However, if there is a difference, then PRT segment takes precedence.
4.5.3.17 OBR-17 Order Callback Phone Number (XTN) 00250
Components: <WITHDRAWN Constituent> ^ <Telecommunication Use Code (ID)> ^ <Telecommunication Equipment Type (ID)> ^ <Communication Address (ST)> ^ <Country Code (SNM)> ^ <Area/City Code (SNM)> ^ <Local Number (SNM)> ^ <Extension (SNM)> ^ <Any Text (ST)> ^ <Extension Prefix (ST)> ^ <Speed Dial Code (ST)> ^ <Unformatted Telephone number (ST)> ^ <Effective Start Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Protection Code (CWE)> ^ <Shared Telecommunication Identifier (EI)> ^ <Preference Order (NM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Shared Telecommunication Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the telephone number for reporting a status or a result using the standard format with extension and/or beeper number when applicable.
4.5.3.18 OBR-18 Placer Field 1 (ST) 00251
Definition: This field is user field #1. Text sent by the placer will be returned with the results.
4.5.3.19 OBR-19 Placer Field 2 (ST) 00252
Definition: This field is similar to placer field #1.
4.5.3.20 OBR-20 Filler Field 1 (ST) 00253
Definition: This field is definable for any use by the filler (diagnostic service).
4.5.3.21 OBR-21 Filler Field 2 (ST) 00254
Definition: This field is similar to filler field #1.
4.5.3.22 OBR-22 Results Rpt/Status Chng - Date/Time (DTM) 00255
Definition: This field specifies the date/time when the results were reported or status changed. This conditional field is required whenever the OBR-25 is valued. This field is used to indicate the date and time that the results are composed into a report and released, or that a status, as defined in ORC-5 order status, is entered or changed. (This is a results field only.) When other applications (such as office or clinical database applications) query the laboratory application for un-transmitted results, the information in this field may be used to control processing on the communications link. Usually, the ordering service would want only those results for which the reporting date/time is greater than the date/time the inquiring application last received results.
4.5.3.23 OBR-23 Charge to Practice (MOC) 00256
Components: <Monetary Amount (MO)> ^ <Charge Code (CWE)>
Subcomponents for Monetary Amount (MO): <Quantity (NM)> & <Denomination (ID)>
Subcomponents for Charge Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the charge to the ordering entity for the studies performed when applicable. The first component is a dollar amount when known by the filler. The second is a charge code when known by the filler (results only).
4.5.3.24 OBR-24 Diagnostic Serv Sect ID (ID) 00257
Definition: This field is the section of the diagnostic service where the observation was performed. If the study was performed by an outside service, the identification of that service should be recorded here. Refer to HL7 Table 0074 - Diagnostic Service Section ID in Chapter 2C, Code Tables, for valid entries.
4.5.3.25 OBR-25 Result Status (ID) 00258
Definition: This field contains the status of results for this order. This conditional field is required whenever the OBR is contained in a report message. It is not required as part of an initial order.
There are two methods of sending status information. If the status is that of the entire order, use ORC-15-order effective date/time and ORC-5-order status. If the status pertains to the order detail segment, use OBR-25-result status and OBR-22-results rpt/status chng - date/time. If both are present, the OBR values override the ORC values.
This field would typically be used in a response to an order status query where the level of detail requested does not include the OBX segments. When the individual status of each result is necessary, OBX-11-observ result status may be used. Refer to HL7 Table 0123 - Result Status in Chapter 2C, Code Tables, for valid entries.
4.5.3.26 OBR-26 Parent Result (PRL) 00259
Components: <Parent Observation Identifier (CWE)> ^ <Parent Observation Sub-identifier (OG)> ^ <Parent Observation Value Descriptor (TX)>
Subcomponents for Parent Observation Identifier (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Parent Observation Sub-identifier (OG): <Original Sub-Identifier (ST)> & <Group (NM)> & <Sequence (NM)> & <Identifier (ST)>
Definition: This field is defined to make it available for other types of linkages (e.g., toxicology). This important information, together with the information in OBR-29-Parent Result Obersvation Identifier and OBR-54 Parent Order, uniquely identifies the parent result's OBX segment related to this order. The value of this OBX segment in the parent result is the organism or chemical species about which this battery reports, or the specific result for which this order or observation is a reflex. For example, if the current battery is an antimicrobial susceptibility, the parent results identified OBX contains a result which identifies the organism on which the susceptibility was run. This indirect linkage is preferred because the name of the organism in the parent result may undergo several preliminary values prior to finalization. In the case of a reflex order, if it is necessary to point to the specific result value for which it is in response, OBR-26 enables pointing to that specific OBX segment.
The third component may be used to record the name of the microorganism identified by the parent result directly. The organism in this case should be identified exactly as it is in the parent culture.
We emphasize that this field does not take the entire result field from the parent. It is meant only for the text name of the organism or chemical subspecies identified. This field is included only to provide a method for linking back to the parent result for those systems that could not generate unambiguous Observation IDs and sub-IDs.
This field is present only when the parent result is identified by OBR-29- Result Observation Identifier or OBR-54, Parent Order, and the parent spawns child orders or results for each of many results. (See Chapter 7 for more details about this linkage.)
A second mode of conveying this information is to use a standard observation result segment (OBX). If more than one organism is present, OBX-4-observation sub-ID is used to distinguish them. In this case, the first OBX with subID N will contain a value identifying the Nth microorganism, and each additional OBX with subID N will contain susceptibility values for a given antimicrobial test on this organism.
4.5.3.27 OBR-27 Quantity/timing
Attention: The OBR-27 element was retained for backward compatibilty only as of v 2.5 and the detail was withdrawn and removed from the standard as of v 2.7.
4.5.3.28 OBR-28 Result Copies To (XCN) 00260
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 27. Additional capabilities are now available through thePRT segment following the OBR using the "RCT" (Results Copy To) value in PRT-4 (Participation) from HL7 Table 912 - Participation in Chapter 2C, Code Tables, and referencing the appropriate participant information using other PRT Fields. The PRT segment is further described in Chapter 7 Section 7.3.4 "PRT - Participation Information Segment".
This field identifies the people who are to receive copies of the results. By local convention, either the ID number or the name may be absent.
4.5.3.29 OBR-29 Parent Result Observation Identifier (EIP) 00261
Components: <Placer Assigned Identifier (EI)> ^ <Filler Assigned Identifier (EI)>
Subcomponents for Placer Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Filler Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field relates a child result to its parent result when a parent child result relationship exists. This field uniquely identifies the order number of the parent result; no other information is required to link the child result with its parent result.
4.5.3.30 OBR-30 Transportation Mode (ID) 00262
Definition: This field identifies how (or whether) to transport a patient, when applicable. Refer to HL7 Table 0124 - Transportation Mode in Chapter 2C, Code Tables, for valid codes.
4.5.3.31 OBR-31 Reason for Study (CWE) 00263
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the code or text using the conventions for coded fields given in the Control chapter (Chapter 2). This is required for some studies to obtain proper reimbursement.
4.5.3.32 OBR-32 Principal Result Interpreter (NDL) 00264
Components: <Name (CNN)> ^ <Start Date/time (DTM)> ^ <End Date/time (DTM)> ^ <Point of Care (IS)> ^ <Room (IS)> ^ <Bed (IS)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Patient Location Type (IS)> ^ <Building (IS)> ^ <Floor (IS)>
Subcomponents for Name (CNN): <ID Number (ST)> & <Family Name (ST)> & <Given Name (ST)> & <Second and Further Given Names or Initials Thereof (ST)> & <Suffix (e.g., JR or III) (ST)> & <Prefix (e.g., DR) (ST)> & <Degree (e.g., MD) (IS)> & <Source Table (IS)> & <Assigning Authority - Namespace ID (IS)> & <Assigning Authority - Universal ID (ST)> & <Assigning Authority - Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.6. The reader is referred to the PRT segment described in Chapter 7.
This field identifies the physician or other clinician who interpreted the observation and is responsible for the report content.
As an example of the use of the PRT segment to replace OBR-32 Primary Result Interpreter, consider the following example:
Harold Hippocrates, MD, as a Primary Interpreter
Provider ID: HHIPP, issued by GoodHealth Hospital (GGH) which is set to expire on 31 December 2011
Dr Hippocrates performed the interpretation from 10:45 to 11 am on 15 April 2011 in the central laboratory (LAB01) at GoodHealth Hospital's main facility (HOSP-MAIN)
In the deprecated form, the message would include (not all of the above information can be represented in this form):
...
OBR|... |HHIPP&Hippocrates&Harold&&&&MD^201104151045^201104151100^LAB01^^^^^^HOSP-MAIN|...<cr>
...
The same information, recast using PRT would appear as:
...
OBR|...<cr>
PRT||AD||PI^Primary Interpreter^HL70443|HHIPP^Hippocrates^Harold^^^^GGH^L^^^PRN^^A^^^G^^20111231^MD||||LAB01^^^^^^HOSP-MAIN||201104151045|201104151100|||<cr>
...
4.5.3.33 OBR-33 Assistant Result Interpreter (NDL) 00265
Components: <Name (CNN)> ^ <Start Date/time (DTM)> ^ <End Date/time (DTM)> ^ <Point of Care (IS)> ^ <Room (IS)> ^ <Bed (IS)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Patient Location Type (IS)> ^ <Building (IS)> ^ <Floor (IS)>
Subcomponents for Name (CNN): <ID Number (ST)> & <Family Name (ST)> & <Given Name (ST)> & <Second and Further Given Names or Initials Thereof (ST)> & <Suffix (e.g., JR or III) (ST)> & <Prefix (e.g., DR) (ST)> & <Degree (e.g., MD) (IS)> & <Source Table (IS)> & <Assigning Authority - Namespace ID (IS)> & <Assigning Authority - Universal ID (ST)> & <Assigning Authority - Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.6. The reader is referred to the PRTsegment used relative to OBR as described in section 4.5.3.32, "Principal Result Interpreter."
This field identifies the clinical observer who assisted with the interpretation of this study.
4.5.3.34 OBR-34 Technician (NDL) 00266
Components: <Name (CNN)> ^ <Start Date/time (DTM)> ^ <End Date/time (DTM)> ^ <Point of Care (IS)> ^ <Room (IS)> ^ <Bed (IS)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Patient Location Type (IS)> ^ <Building (IS)> ^ <Floor (IS)>
Subcomponents for Name (CNN): <ID Number (ST)> & <Family Name (ST)> & <Given Name (ST)> & <Second and Further Given Names or Initials Thereof (ST)> & <Suffix (e.g., JR or III) (ST)> & <Prefix (e.g., DR) (ST)> & <Degree (e.g., MD) (IS)> & <Source Table (IS)> & <Assigning Authority - Namespace ID (IS)> & <Assigning Authority - Universal ID (ST)> & <Assigning Authority - Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.6. The reader is referred to the PRTsegment used relative to OBR as described in section 4.5.3.32, "Principal Result Interpreter."
This field identifies the performing technician.
4.5.3.35 OBR-35 Transcriptionist (NDL) 00267
Components: <Name (CNN)> ^ <Start Date/time (DTM)> ^ <End Date/time (DTM)> ^ <Point of Care (IS)> ^ <Room (IS)> ^ <Bed (IS)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Patient Location Type (IS)> ^ <Building (IS)> ^ <Floor (IS)>
Subcomponents for Name (CNN): <ID Number (ST)> & <Family Name (ST)> & <Given Name (ST)> & <Second and Further Given Names or Initials Thereof (ST)> & <Suffix (e.g., JR or III) (ST)> & <Prefix (e.g., DR) (ST)> & <Degree (e.g., MD) (IS)> & <Source Table (IS)> & <Assigning Authority - Namespace ID (IS)> & <Assigning Authority - Universal ID (ST)> & <Assigning Authority - Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field is retained for backward compatibility only as of v 2.6. The reader is referred to the PRTsegment used relative to OBR as described in section 4.5.3.32, "Principal Result Interpreter."
This field identifies the report transcriber.
4.5.3.36 OBR-36 Scheduled Date/Time (DTM) 00268
Definition: This field is the date/time the filler scheduled an observation, when applicable (e.g., action code in OBR-11-specimen action code = "S"). This is a result of a request to schedule a particular test and provides a way to inform the placer of the date/time a study is scheduled (result only).
4.5.3.37 OBR-37 Number of Sample Containers (NM) 01028
Definition: This field identifies the number of containers for a given sample. For sample receipt verification purposes; may be different from the total number of samples which accompany the order.
4.5.3.38 OBR-38 Transport Logistics of Collected Sample (CWE) 01029
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the means by which a sample reaches the diagnostic service provider. This information is to aid the lab in scheduling or interpretation of results. Possible answers: routine transport van, public postal service, etc. If coded, requires a user-defined table.
4.5.3.39 OBR-39 Collector's Comment (CWE) 01030
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is for reporting additional comments related to the sample. If coded, requires a user-defined table. If only free text is reported, it is placed in the second component with a null in the first component, e.g., ^difficulty clotting after venipuncture and ecchymosis.
4.5.3.40 OBR-40 Transport Arrangement Responsibility (CWE) 01031
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is an indicator of who is responsible for arranging transport to the planned diagnostic service. Examples: Requester, Provider, Patient. If coded, requires a user-defined table.
4.5.3.41 OBR-41 Transport Arranged (ID) 01032
Definition: This field is an indicator of whether transport arrangements are known to have been made. Refer to HL7 Table 0224 - Transport Arranged in Chapter 2C, Code Tables, for valid codes.
4.5.3.42 OBR-42 Escort Required (ID) 01033
Definition: This field is an indicator that the patient needs to be escorted to the diagnostic service department. Note: The nature of the escort requirements should be stated in OBR-43-planned patient transport comment. See HL7 Table 0225 - Escort Required in Chapter 2C, Code Tables, for valid values.
4.5.3.43 OBR-43 Planned Patient Transport Comment (CWE) 01034
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the code or free text comments on special requirements for the transport of the patient to the diagnostic service department. If coded, requires a user-defined table.
4.5.3.44 OBR-44 Procedure Code (CNE) 00393
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains a unique identifier assigned to the procedure, if any, associated with the charge. Refer to Externally-defined table 0088 - Procedure code in Chapter 2C, Code Tables, for suggested values. This field is a coded data type for compatibility with clinical and ancillary systems.
As of version 2.6, applicable external coding systems include those in the referenced table. If the code set used is in the referenced table, then the coding scheme designation in the table shall be used.
4.5.3.45 OBR-45 Procedure Code Modifier (CNE) 01316
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the procedure code modifier to the procedure code reported in OBR-44-procedure code, when applicable. Procedure code modifiers are defined by regulatory agencies such as CMS and the AMA. Multiple modifiers may be reported. The modifiers are sequenced in priority according to user entry. In the USA, this is a requirement of the UB and the 1500 claim forms. Multiple modifiers are allowed and the order placed on the form affects reimbursement. Refer to Externally- defined table 0340 - Procedure code modifier in Chapter 2C, Code Tables, for suggested values.
Usage Rule: This field can only be used if OBR-44 - procedure code contains certain procedure codes that require a modifier in order to be billed or performed. For example, HCPCS codes that require a modifier to be precise.
As of version 2.6, applicable external coding systems include those in the referenced table. If the code set used is in the referenced table, then the coding scheme designation in the table shall be used.
4.5.3.46 OBR-46 Placer Supplemental Service Information (CWE) 01474
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains supplemental service information sent from the placer system to the filler system for the universal procedure code reported in OBR-4 Universal Service ID. This field will be used to provide ordering information detail that is not available in other specific fields in the OBR segment. Multiple supplemental service information elements may be reported. Refer to User-defined Table 0411 - Supplemental service information values in Chapter 2C, Code Tables.
This field can be used to describe details such as whether study is to be done on the right or left, for example, where the study is of the arm and the order master file does not distinguish right from left, or whether the study is to be done with or without contrast (when the order master file does not make such distinctions).
4.5.3.47 OBR-47 Filler Supplemental Service Information (CWE) 01475
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains supplemental service information sent from the filler system to the placer system for the procedure code reported in OBR-4 Universal Service ID. This field will be used to report ordering information detail that is not available in other specific fields in the OBR segment. Typically it will reflect the same information as was sent to the filler system in OBR-46-Placer supplemental service information unless the order was modified, in which case the filler system will report what was actually performed using this field. Multiple supplemental service information elements may be reported. Refer to User-Defined Table 0411 - Supplemental Service Information Values in Chapter 2C, Code Tables.
This field can be used to describe details such as whether study is to be done on the right or left, for example, where the study is of the arm and the order master file does not distinguish right from left, or whether the study is to be done with or without contrast (when the order master file does not make such distinctions).
4.5.3.48 OBR-48 Medically Necessary Duplicate Procedure Reason (CWE) 01646
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is used to document why the procedure found in OBR-44 - Procedure Code is a duplicate of one ordered/charged previously for the same patient within the same date of service and has been determined to be medically necessary. The reason may be coded or it may be a free text entry.
This field is intended to provide financial systems information on who to bill for duplicate procedures.
Refer to User-Defined Table 0476 - Medically Necessary Duplicate Procedure Reason in Chapter 2C, Code Tables, for suggested values.
4.5.3.49 OBR-49 Result Handling (CWE) 01647
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Transmits information regarding the handling of the result. For example, an order may specify that the result (e.g., an x-ray film) should be given to the patient for return to the requestor. Refer to HL7 Table 0507 - Observation Result Handling in Chapter 2C, Code Tables, for values. If this field is not populated or if it includes value "CC^Copies Requested", then routine handling is implied and PRT segments assocatied with this OBR with PRT-4 value of "RCT^Result Copies To" identify additional recipients for the results. When this field includes the value "BCC^Blind Copy", those PRT segments, which are included in the order message and in the observation result message sent to the requestor, shall not be included in the observation result messages sent to the copied recipients.
4.5.3.50 OBR-50 Parent Universal Service Identifier (CWE) 02286
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is retained for backward compatibility only as of v 2.7. Note that OBR-50 has been deprecated in v 2.7 to enable message developers to adjust and be prepared for supporting the intended 1:1 relationship between Placer/Filler Order Number and Universal Service Identifier.
This field contains the identifier code for the parent order, as identified in ORC-8 Parent and/or OBR-29 Parent (if present), which caused this observation/test/battery to be performed. This can be based on local and/or "universal" codes. HL7 recommends the "universal" service identifier.
Note that ORC-8 Parent and/or OBR-29 Parent, does not have to be present for OBR-50 to be used. However, absence of ORC-8 Parent and/or OBR-29 Parent introduces potential ambiguity of the actual order being referenced.
Note that ORC-8 Parent and OBR-29 Parent identify an individual parent order (e.g., OBR) for ORC-31 Parent Universal Service Identifier and OBR-50 Parent Universal Service Identifier.
ORC-31 - parent universal service identifier is the same as OBR-50 - parent universal service identifier. If both fields are valued, they must contain the same value.
4.5.3.51 OBR-51 Observation Group ID (EI) 02307
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The Observation Group ID is the identifier assigned by the producer of a result to uniquely identify the results associated with this OBR segment. The Observation Group ID is intended to remain the same regardless of the change in status to the result (i.e., it is not a snapshot ID). This field is intended to promote forward compatibility with HL7 V3.
4.5.3.52 OBR-52 Parent Observation Group ID (EI) 02308
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The Parent Observation Group ID field relates this child OBR to its parent OBR segment using the Observation Group ID of the parent result.
4.5.3.53 OBR-53 Alternate Placer Order Number (CX) 03303
Components: <ID Number (ST)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Effective Date (DT)> ^ <Expiration Date (DT)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field enables a shorter number to be communicated that is unique within other identifiers.
4.5.3.54 OBR-54 Parent Order (EIP) 00222
Components: <Placer Assigned Identifier (EI)> ^ <Filler Assigned Identifier (EI)>
Subcomponents for Placer Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Filler Assigned Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field relates a child order to its parent order when a parent child order relationship exists. The parent child order mechanism is described in HL7 Table 0119 - Order Control Codes in Chapter 2C, Code Tables, under order control code PA. This field uniquely identifies the parent order; no other information is required to link the child order with its parent order.
The first component has the same format as ORC-2-placer order number (Section â??4.5.3.2, "Placer Order Number (EI) 00216"). The second component has the same format as ORC-3-filler order number (Section â??4.5.3.3, "Filler Order Number (EI) 00217"). The components of the placer order number and the filler order number are transmitted in sub-components of the two components of this field.
Note that ORC-8 - Parent Order is equivalent to OBR-54-Parent Order, but neither one is the same as OBR-29-Parent Result Obersvation Identifier .
Condition: Where the message has matching ORC/OBR pairs, ORC-8 and OBR-54 must carry the same value.
4.5.4 TQ1 - Timing/Quantity Segment
The TQ1 segment is used to specify the complex timing of events and actions such as those that occur in order management and scheduling systems. This segment determines the quantity, frequency, priority and timing of a service. By allowing the segment to repeat, it is possible to have service requests that vary the quantity, frequency and priority of a service request over time.
Use cases showing when TQ1 may need to repeat:
-
Cardiac enzymes STAT and then q 4 hours.
-
Streptokinase studies, draw 1st Stat and run Stat, then draw q 4 hours and run Stat.
-
Gentamicin 100mg Now and 80mg q12h second dose (First 80mg dose) given exactly 12 hours after the 100mg dose. (Might be 2 service requests.)
-
Activase 15mg bolus Stat then 50mg over 30 minutes, then 35mg over the next 60 minutes. (Might be 2 service requests.)
-
Imodium 4mg (2 caps) po initially, then 2mg (1cap) after each unformed stool to a maximum of 16 mg per day. (Might be 2 service requests.)
-
Zithromax 500mg (2tabs) po on the first day then 250mg (1tab) po qd for 5 days. (Might be 2 service requests.)
-
Zyban (Bupropion) Start 150mg po qam x 3 days, then increase to 150mg po bid for 7 to 12 weeks.
-
Colchicine 1mg (2 tabs) po now then 1 tablet q1 to 2 hours until pain relief or undesirable side effects (Diarrhea, GI upset). (Might be 2 service requests.)
-
doxycylcine 100mg po bid on the first day then 100mg po qd.
-
scopolamine, xxx mg, 1 hour before surgery. Relative time = -1^hour, priority = P (preop), or alternately repeat pattern = P1H^Preop, 1 Hour before Surgery^99LocalCode, Relative time would be empty and priority would be P (preop).
HL7 Attribute Table - TQ1 - Timing/Quantity
4.5.4.0 TQ1 field definitions
4.5.4.1 TQ1-1 Set ID - TQ1 (SI) 01627
Definition: For the first timing specification transmitted, the sequence number shall be 1; for the second timing specification, it shall be 2; and so on.
4.5.4.2 TQ1-2 Quantity (CQ) 01628
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field specifies the numeric quantity of the service that should be provided at each service interval. For example, if two blood cultures are to be obtained every 4 hours, the quantity would be '2', or if three units of blood are to be typed and cross-matched, the quantity would be '3'. The default value for this field is '1'.
If multiple identical services are to be requested, it is strongly recommended that multiple service requests be placed, giving each service request its own unique placer/filler number.
4.5.4.3 TQ1-3 Repeat Pattern (RPT) 01629
Components: <Repeat Pattern Code (CWE)> ^ <Calendar Alignment (ID)> ^ <Phase Range Begin Value (NM)> ^ <Phase Range End Value (NM)> ^ <Period Quantity (NM)> ^ <Period Units (CWE)> ^ <Institution Specified Time (ID)> ^ <Event (ID)> ^ <Event Offset Quantity (NM)> ^ <Event Offset Units (CWE)> ^ <General Timing Specification (GTS)>
Subcomponents for Repeat Pattern Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Period Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Event Offset Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The repeating frequency with which the treatment is to be administered. It is similar to the frequency and SIG code tables used in order entry systems.
This field may be repeated to build up more complex repeat patterns. For example, daily at bedtime can be represent as "|QD~HS|".
When the quantity timing specification must change to a different repeat pattern after some period of time, a new TQ1 segment must be used to show the new repeat pattern. Note that the end date of the current TQ1 will show when the current timing specification ends, and the start date of the next TQ1 shows when the new timing specification begins. The Conjunction field, TQ1-12 determines if the next TQ1 segment is to be performed sequentially or in parallel.
4.5.4.4 TQ1-4 Explicit Time (TM) 01630
Definition: This field explicitly lists the actual times referenced by the code in TQ1-3. This field will be used to clarify the TQ1-3 in cases where the actual administration times vary within an institution. If the time of the service request spans more than a single day, this field is only practical if the same times of administration occur for each day of the service request. If the actual start time of the service request (as given by TQ1-7) is after the first explicit time, the first administration is taken to be the first explicit time after the start time. In the case where the patient moves to a location having a different set of explicit times, the existing service request may be updated with a new quantity/timing segment showing the changed explicit times.
Usage Note: This field is not valued if a Repeat Pattern is not present.
4.5.4.5 TQ1-5 Relative Time and Units (CQ) 01631
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field is used to define the interval between schedules for service request or bottle records. If this field contains a value, it overrides any value in the explicit time interval field. The units component of the CQ data type is constrained to units of time.
Examples:
TQ1|1|1|Q1H||60^min&&ANS+ - Q1H is defined with an interval between services of 60 minutes
TQ1|1|1|Q6H||6^hr&&ANS+ - Q6H is defined with an interval between services of 6 hours
TQ1|1|1|QD||1^d&&ANS+ - QD is defined with an interval between services of 1 day
4.5.4.6 TQ1-6 Service Duration (CQ) 01632
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the duration for which the service is requested.
The quantity component of this field must be a positive, non-zero number. The unit's portion of this field is constrained to units of time.
Example: Whirlpool twenty minutes three times per day for 3 days. Three days is the service duration.
TQ1|1||TID|||3^d&&ANS+||||||20^min&&ANS+|9<cr>
4.5.4.7 TQ1-7 Start Date/Time (DTM) 01633
Definition: This field may be specified by the requester, in which case it indicates the earliest date/time at which the services should be started. In many cases, however, the start date/time will be implied or will be defined by other fields in the service request record (e.g., urgency - STAT). In such a case, this field will be empty.
The filling service will often record a value in this field after receipt of the service request, however, and compute an end time on the basis of the start date/time for the filling service's internal use.
4.5.4.8 TQ1-8 End Date/Time (DTM) 01634
Definition: When filled in by the requester of the service, this field should contain the latest date/time that the service should be performed. If it has not been performed by the specified time, it should not be performed at all. The requester may not always fill in this value, yet the filling service may fill it in on the basis of the instruction it receives and the actual start time.
Regardless of the value of the end date/time, the service should be stopped at the earliest of the date/times specified by either the duration or the end date/time.
4.5.4.9 TQ1-9 Priority (CWE) 01635
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field describes the urgency of the request. If this field is blank, the default is R. Refer to User-Defined Table 0485 - Extended Priority Codes in Chapter 2C, Code Tables, for suggested values.
4.5.4.10 TQ1-10 Condition Text (TX) 01636
Definition: This is a free text field that describes the conditions under which the drug is to be given. For example, "PRN pain," or "to keep blood pressure below 110."
The presence of text in this field should be taken to mean that human review is needed to determine the how and/or when this drug should be given.
For complex codified conditions see the TQ2 segment below.
4.5.4.11 TQ1-11 Text Instruction (TX) 01637
Definition: This field is a full text version of the instruction (optional).
4.5.4.12 TQ1-12 Conjunction (ID) 01638
Definition: This field indicates that a second TQ1 segment is to follow. Refer To HL7 Table 0472 - TQ Conjunction ID in Chapter 2C, Code Tables, for allowed values.
For continuous or periodic services, the point at which the service is actually stopped is determined by the field TQ1-8 end date/time and TQ1-6 duration, whichever indicates an earlier stopping time. Ordinarily, only one of these fields would be present.
Condition Rule: If the TQ1 segment is repeated in the message, this field must be populated with the appropriate Conjunction code indicating the sequencing of the following TQ1 segment.
4.5.4.13 TQ1-13 Occurrence Duration (CQ) 01639
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the duration for which a single performance of a service is requested. The quantity component of this field must be a positive, non-zero number when populated. The units component is constrained to be units of time.
Example: Whirlpool twenty minutes three times per day for three days. Twenty minutes is the occurrence duration.
TQ1|1||TID|||3^d&&ANS+||||||20^min&&ANS+|9<cr>
4.5.4.14 TQ1-14 Total Occurrences (NM) 01640
Definition: This field contains the total number of occurrences of a service that should result from this service request. If both the end date/time (TQ1-8) and the total occurrences are valued and the occurrences would extend beyond the end date/time, then the end date/time takes precedence. Otherwise the number of occurrences takes precedence.
Example: Whirlpool twenty minutes three times per day for three days. The total occurrences would be 9.
TQ1|1||TID|||3^d&&ANS+||||||20^min&&ANS+|9<cr>
4.5.5 TQ2 - Timing/Quantity Relationship
The TQ2 segment is used to form a relationship between the service request the TQ1/TQ2 segments are associated with, and other service requests. The TQ2 segment will link the current service request with one or more other service requests.
There are many situations, such as the creation of a service request for a group of intravenous (IV) solutions, where the sequence of the individual intravenous solutions (each a service in itself) needs to be specified, e.g., hyperalimentation with multi-vitamins in every third bottle.
There are other situations where part of the service request's instructions contains a results condition of some type, such as "PRN pain." There is currently a free text "condition" field of TQ1-10 - Condition text which allows any condition to be specified. However, to support a fully encoded version of service request sequencing, or results condition, the TQ2, Timing/Quantity Relationship segment has been defined.
HL7 Attribute Table - TQ2 - Timing/Quantity Relationship
|
SEQ
|
LEN
|
C.LEN
|
DT
|
OPT
|
RP/#
|
TBL#
|
ITEM#
|
ELEMENT NAME
|
DB Ref. |
|
1
|
1..4
|
|
SI
|
O
|
|
|
01648
|
Set ID - TQ2
|
DB |
|
2
|
1..1
|
|
ID
|
O
|
|
0503
|
01649
|
Sequence/Results Flag
|
DB |
|
3
|
|
|
EI
|
C
|
Y
|
|
01650
|
Related Placer Number
|
DB |
|
4
|
|
|
EI
|
C
|
Y
|
|
01651
|
Related Filler Number
|
DB |
|
5
|
|
|
EI
|
C
|
Y
|
|
01652
|
Related Placer Group Number
|
DB |
|
6
|
2..
|
|
ID
|
C
|
|
0504
|
01653
|
Sequence Condition Code
|
DB |
|
7
|
1..1
|
|
ID
|
C
|
|
0505
|
01654
|
Cyclic Entry/Exit Indicator
|
DB |
|
8
|
|
|
CQ
|
O
|
|
|
01655
|
Sequence Condition Time Interval
|
DB |
|
9
|
|
10=
|
NM
|
O
|
|
|
01656
|
Cyclic Group Maximum Number of Repeats
|
DB |
|
10
|
1..1
|
|
ID
|
C
|
|
0506
|
01657
|
Special Service Request Relationship
|
DB |
TQ2 Usage notes:
-
Cyclic placer service request groups
To implement a cyclic group of four IV service requests using the parent/child paradigm, the parent specifies a custom group of IVs, and the following occurs:
-
TQ2 of the second child service request specifies that it follow the first child service request.
-
TQ2 of the third child service request specifies that it follow the second child service request.
-
TQ2 of the fourth child service request specifies that it follow the third service request.
To repeat the group of four child service requests in a cyclic manner, the following occurs:
This scheme allows the following to be tracked:
-
The status of the whole group of service requests to be reported back at the level of the parent service request.
-
The status for each individual IV service request by following the status of the corresponding child service request.
Separate Service requests example:
-
The same group of service requests can be sent as a group of four service requests (without a common parent), linked only by the data in their quantity/timing fields. In this case, there is no convenient HL7 method of transmitting the service request status of the group as a whole without transmitting the status of each of the four separate service requests.
-
Inheritance of service request status
Cancellation/discontinuation/hold service request control events:
-
This logic implies the normal execution of the referenced predecessor service request. Thus a cancel (or discontinuation or hold) of a predecessor service request implies the cancellation (or discontinuation or hold) of all subsequent service requests in the chain.
-
If the referenced service request has been canceled (or discontinued or held), the current service request inherits that same status.
-
In the case of hold, the removal of the hold of the predecessor implies a removal of the hold for the given service request (which can then be executed according to the specification in the TQ2 segment).
4.5.5.0 TQ2 field definitions
4.5.5.1 TQ2-1 Set ID - TQ2 (SI) 01648
Definition: For the first timing specification transmitted, the sequence number shall be 1; for the second timing specification, it shall be 2; and so on.
4.5.5.2 TQ2-2 Sequence/Results Flag (ID) 01649
Definition: This flag defines the sequencing relationship between the current service request, and the related service request(s) specified in this TQ2 segment. See HL7 Table 0503 - Sequence/Results Flag in Chapter 2C, Code Tables, for values. If not value is present, the S - Sequential is the default value.
4.5.5.3 TQ2-3 Related Placer Number (EI) 01650
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The placer numbers of the service request(s) to which this TQ2 segment links the current service request. This field should be populated with the appropriate "Placer number" from the current service request. For orders, the Placer Order Number from ORC-2 is the appropriate "Placer number". Repeats of this field indicate the current service request is related to multiple service requests.
Conditional Rule: At least one of TQ2-3, TQ2-4, TQ2-5 must contain a value.
4.5.5.4 TQ2-4 Related Filler Number (EI) 01651
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The filler numbers of the service request(s) to which this TQ2 segment links the current service request. This field should be populated with the appropriate "Filler number" from the current service request. For orders, the Filler Order Number from ORC-3 is the appropriate "Filler number". Repeats of this field indicate the current service request is related to multiple service requests.
Conditional Rule: At least one of TQ2-3, TQ2-4, TQ2-5 must contain a value.
4.5.5.5 TQ2-5 Related Placer Group Number (EI) 01652
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The placer group numbers of the service request(s) to which this TQ2 segment links the current service request. This field should be populated with the appropriate "Placer group number" from the current service request. For orders, the Placer Group Number from ORC-4 is the appropriate "Placer group number". Repeats of this field indicate that the current service request is related to multiple groups of service requests.
Conditional Rule: At least one of TQ2-3, TQ2-4, TQ2-5 must contain a value.
4.5.5.6 TQ2-6 Sequence Condition Code (ID) 01653
Definition: Defines the relationship between the start/end of the related service request(s) (from TQ2-3, TQ2-4, or TQ2-5) and the current service request from ORC-2, 3 or 4. See HL7 Table 0504 - Sequence Condition Code in Chapter 2C Code Tables, for allowed values.
Conditional Rule: Either this field or TQ2-10 must be present.
4.5.5.7 TQ2-7 Cyclic Entry/Exit Indicator (ID) 01654
Definition: Indicates if this service request is the first, last, service request in a cyclic series of service requests. If null or not present, this field indicates that the current service request is neither the first or last service request in a cyclic series of service requests. Refer to HL7 Table 0505 - Cyclic Entry/Exit Indicator in Chapter 2C, Code Tables, for allowed values.
Conditional Rule: Should not be populated when TQ2-2 (Sequence/Results Flag) is not equal to a 'C' (cyclic service request).
Example of TQ2 - 6, 7, & 8 Usage:
|
Example
|
Translation
|
|
...|ES|*|+10^min|...
|
translates to: execute this service request the first time without evaluating the condition specified in the TQ2 segment; but repeat only its execution when the specified external service request's start or finish date/time has met this condition. This specification generates a repetition of the service request for each iteration of the cycle.
|
Note: This requires that the requesting application be able to specify the placer/filler/placer group number of the last service request in the cycle in the first service request's quantity/timing specification.
4.5.5.8 TQ2-8 Sequence Condition Time Interval (CQ) 01655
Components: <Quantity (NM)> ^ <Units (CWE)>
Subcomponents for Units (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: Defines the interval of time between the start/end of the related service request(s) and the start/end of the current service request. The unit's component is constrained to units of time. If this field is not populated, then there should be no interruption between start/ending the current service request, and the related service request(s).
4.5.5.9 TQ2-9 Cyclic Group Maximum Number of Repeats (NM) 01656
Definition: The maximum number of repeats for a cyclic group.
The total number of repeats is constrained by the end date/time of the last repeat or the end date/time of the parent, whichever is first. For example, if the total number or repeats is valued at 10 and the group has already repeated 5 times, the current order will not be repeated again if either the current order, or the prior order in the cycle, has reached its end date/time.
This field is meaningful only when TQ2-2 Sequence/Results Flag is valued with 'C'. However, even in this case this field is optional.
4.5.5.10 TQ2-10 Special Service Request Relationship (ID) 01657
Definition: This defines an additional or alternate relationship between this service request and other service requests. Its primary intended use is for Pharmacy administration service requests, but it may be useful for other domains. See HL7 Table 0506 - Service Request Relationship in Chapter 2C, Code Tables, for allowed values.
Conditional Rule: Either this field or TQ2-6 must be present.
4.5.6 IPC - Imaging Procedure Control Segment
The IPC segment contains information about tasks that need to be performed in order to fulfill the request for imaging service. The information includes location, type and instance identification of equipment (acquisition modality) and stages (procedure steps).
Note: References, field names and definitions in this section were developed in collaboration with DICOM with the goal of keeping HL7 transmission of imaging procedure control information consistent with the DICOM Standard, available at http://medical.nema.org.
HL7 Attribute Table - IPC - Imaging Procedure Control Segment
|
SEQ
|
LEN
|
C.LEN
|
DT
|
OPT
|
RP/#
|
TBL#
|
ITEM #
|
ELEMENT NAME
|
DB Ref. |
|
1
|
|
|
EI
|
R
|
|
|
01330
|
Accession Identifier
|
DB |
|
2
|
|
|
EI
|
R
|
|
|
01658
|
Requested Procedure ID
|
DB |
|
3
|
|
|
EI
|
R
|
|
|
01659
|
Study Instance UID
|
DB |
|
4
|
|
|
EI
|
R
|
|
|
01660
|
Scheduled Procedure Step ID
|
DB |
|
5
|
|
|
CWE
|
O
|
|
9999
|
01661
|
Modality
|
DB |
|
6
|
|
|
CWE
|
O
|
Y
|
9999
|
01662
|
Protocol Code
|
DB |
|
7
|
|
|
EI
|
O
|
|
|
01663
|
Scheduled Station Name
|
DB |
|
8
|
|
|
CWE
|
O
|
Y
|
9999
|
01664
|
Scheduled Procedure Step Location
|
DB |
|
9
|
|
16=
|
ST
|
O
|
|
|
01665
|
Scheduled Station AE Title
|
DB |
4.5.6.0 IPC field definitions
4.5.6.1 IPC-1 Accession Identifier (EI) 01330
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: A workflow-management IDIS generated number that identifies the Filler Order for an Imaging Service (Imaging Service Request). This identifier corresponds one-to-one to the Order Filler number but is used in internal tracking of the work by the IDIS and in communication between IDIS within the department. It also has specific requirements to assure its compatibility with DICOM. It is a case of the Entity Identifier data type (section 2.A.28). Its first component is a string that identifies the Imaging Service Request. A limit of sixteen (16) characters is required to allow compatibility with DICOM. See DICOM Standard Part 3 for further details on DICOM Attribute (0008,0050) that conveys information identical to the component one of this field.
An IDIS that performs functions of the workflow management for a department may accept a single Placer Order that gives rise to one or more Filler Orders-Imaging Service Requests. For example, an IDIS may receive an order for an X-ray examination of the patient daily at 8 am for the next three days. For the purposes of fulfilling the Placer Order, it will identify each of the daily exams either as a separate Filler Order or parts of a single Filler Order. Correspondingly, it will assign one or more Filler Order numbers associated with the order. For each of the Filler Order numbers, it will assign a unique Accession Number.
Each of the Imaging Service Requests may contain one or more Requested Procedures that it will identify with the Requested Procedure ID. The Requested Procedure is the most granular unit of work that may lead to the creation of the procedure report. Each procedure report contributes to the results for the order. In the example mentioned above, each of the daily examinations will require a separate diagnostic report, hence each of them will be treated as a separate Requested Procedure. Depending on the treatment of the order by the IDIS, it will either link all Requested Procedures to a single Filler Order-Imaging Service Request, or link each Requested Procedure to its own Imaging Service Request. Exact type of requested procedure is conveyed by the coded values in OBR-44 Procedure Code and OBR-45 Procedure Code modifier for each procedure. Note that in case of multiple Requested Procedures corresponding to one order, each procedure may have different code.
To support communication with the instances of equipment in a department (acquisition modalities), IDIS will also generate the Study Instance UID, a globally unique identifier for each Requested Procedure. This identifier will be used by acquisition modalities to identify all generated images and other DICOM objects related to this Requested Procedure. Note that, unlike the Study Instance UID, the Requested Procedure ID must only be unique within the scope of the encompassing Imaging Service Request identified by an Accession Number.
Each of the Requested Procedures may be further broken down by the IDIS into the Scheduled Procedure Steps based on the timing and equipment requirements. Each step is identified with the Scheduled Procedure Step ID. A single Procedure Step may only be performed on a single type and instance of the equipment. Thus, while the Requested Procedure may identify multi-modality examination (such as ones common in Nuclear Medicine), a single Procedure Step shall correspond to the operations performed on a single modality.
The example of the hierarchy of Imaging Service Request, Requested Procedure and Scheduled Procedure Step is depicted in a figure 4-6. Identifiers of the entities are represented by the field names stated in square brackets.
Figure 4-6. Hierarchy of Imaging Service Request, Requested Procedure and Scheduled Procedure Step
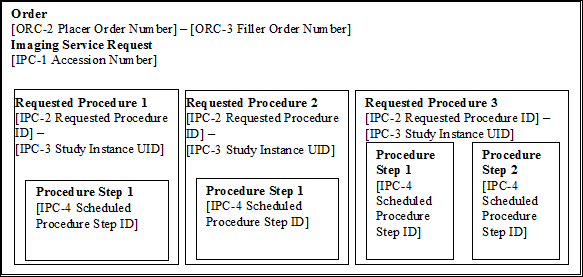
The full hierarchy constitutes the context that will be shared between all IDIS within a department in a course of order fulfillment.
Each OMI message shall convey information about Requested Procedure(s) pertaining to one order. A pair of Segments ORC/OBR shall correspond to each requested procedure. If the Requested Procedure is comprised of multiple Procedure Steps, multiple IPC segments shall be included for each ORC/OBR pair in the message. Value of the IPC-1 field shall be identical in all IPC segments.
Considering the preceding example of X-ray examinations on subsequent days with two different steps identified for the last Requested Procedure and examinations to be performed at the site, "RADIOLOGY", the communication of the information using OMI message may look like the following:
MSH|...<cr>
PID|...<cr>
ORC|NW|...<cr>
OBR|1|X1234^HIS|R578^RIS|56782^X-Ray Chest|...|XPA^X-Ray Chest PA|...<cr>
IPC|A345^RIS|P1234^RIS|1.2.840.1234567890.3456786.1^RIS|SPS1^RIS|CR|SXPA^Chest PA||RADIOLOGY|<cr>
ORC|NW|...<cr>
OBR|2|X1234^HIS|R578^RIS|56782^X-Ray Chest|...|XPA^X-Ray Chest PA|...<cr>
IPC|A345^RIS|P1235^RIS|1.2.840.1234567890.3456786.2^RIS|SPS1^RIS|CR|SXPA^Chest PA||RADIOLOGY|<cr>
ORC|NW|...<cr>
OBR|3|X1234^HIS|R578^RIS|56782^X-Ray Chest|...|XPALAT^X-Ray Chest PA and Lateral|...<cr>
IPC|A345^RIS|P1236^RIS|1.2.840.1234567890.3456786.3^RIS|SPS1^RIS|CR|SXPA^Chest PA||RADIOLOGY|<cr>
IPC|A345^RIS|P1236^RIS|1.2.840.1234567890.3456786.3^RIS|SPS2^RIS|CR|SXLAT^Chest Lat||RADIOLOGY|<cr>
4.5.6.2 IPC-2 Requested Procedure ID (EI) 01658
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is the identifier of the Requested Procedure that the workflow management IDIS selected to perform as a part of the order for the imaging service. It is a case of the Entity Identifier data type (section 2.A.28). The first component of this field is a string that identifies the Requested Procedure. A limit of sixteen (16) characters is required to allow compatibility with DICOM. This string must uniquely identify the Requested Procedure within the scope of the order (as specified by accession number). This uniqueness must persist over time. See DICOM Standard Part 3 for further details on DICOM Attribute (0040,0001) that conveys information identical to the component one of this field.
The second through fourth components contain the ID of the workflow management IDIS, in the form of the HD data type (see section 2.A.36, "HD - hierarchic designator"). The second component is a user-defined coded value that uniquely defines the application from other applications on the network. A limit of five (5) characters is suggested but not required. The second component of the Requested Procedure number always identifies the actual filler of an order.
A Requested Procedure is an instance of a Procedure of a given Procedure Type. An instance of a Requested Procedure includes all of the items of information that are specified by an instance of a Procedure Plan that is selected for the Requested Procedure by the imaging service provider. This Procedure Plan is defined by the imaging service provider on the basis of the Procedure Plan templates associated with the considered Procedure Type. An Imaging Service Request may include requests for several different Requested Procedures. The purpose of this entity is to establish the association between Imaging Service Requests and Procedure Types, to convey the information that belongs to this association and to establish the relationships between Requested Procedures and the other entities that are needed to describe them. A single Requested Procedure of one Procedure Type is the smallest unit of service that can be requested, reported, coded and billed. Performance of one instance of a Requested Procedure is specified by exactly one Procedure Plan. A Requested Procedure leads to one or more Scheduled Procedure Steps involving Protocols as specified by a Procedure Plan. A Requested Procedure may involve one or more pieces of equipment.
Each OMI message shall convey information about Requested Procedure(s) pertaining to one order. Pair of Segments ORC/OBR shall correspond to each requested procedure. If the Requested Procedure is comprised of multiple Procedure Steps, multiple IPC segments shall be included for each ORC/OBR pair in the message. In this case, the value of the IPC-2 field shall be identical in all IPC segments related to the same Requested Procedure.
4.5.6.3 IPC-3 Study Instance UID (EI) 01659
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: Globally unique identifier assigned by the workflow management IDIS to the Imaging Study under which all images and other DICOM objects produced in the course of the Requested Procedure shall be collected. It is a case of the Entity Identifier data type (section 2.A.28). Its first component is a string that identifies the Study. A limit of sixty-four (64) characters is required to allow compatibility with DICOM. See DICOM Standard Part 3 for further details on DICOM Attribute (0020,000D) that conveys information identical to component one of this field. The second through fourth components contain the ID of the workflow management IDIS, in the form of the HD data type (see section 2.A.36, "HD - hierarchic designator"). The second component is a user-defined coded value that uniquely defines the application from other applications on the network. A limit of five (5) characters is suggested but not required. The second component of the Study Instance UID always identifies the actual filler of an order.
Each OMI message shall convey information about Requested Procedure(s) pertaining to one order. Pair of Segments ORC/OBR shall correspond to each requested procedure. If the Requested Procedure is comprised of multiple Procedure Steps, multiple IPC segments shall be included for each ORC/OBR pair in the message. In this case, the value of the IPC-3 field shall be identical in all IPC segments related to the same Requested Procedure.
4.5.6.4 IPC-4 Scheduled Procedure Step ID (EI) 01660
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is the identifier of a particular Procedure Step (sub-procedure) of the Requested Procedure that the workflow management IDIS selected to perform as a part of the order for imaging service. It is a case of the Entity Identifier data type (section 2.A.28). Its first component is a string that identifies the Procedure Step. A limit of sixteen (16) characters is required to allow compatibility with DICOM. This string must uniquely identify the Procedure Step within the scope of the Requested Procedure. This uniqueness must persist over time. See DICOM Standard Part 3 for further details on DICOM Attribute (0040,0009) that conveys information identical to the component one of this field.
The second through fourth components contain the ID of the workflow management IDIS, in the form of the HD data type (see section 2.A.36, "HD - hierarchic designator"). The second component is a user-defined coded value that uniquely defines the application from other applications on the network. A limit of five (5) characters is suggested but not required. The second component of the Requested Procedure number always identifies the actual filler of an order.
A Procedure Step is an arbitrarily defined scheduled unit of service, which is specified by the Procedure Plan for a Requested Procedure. A Procedure Step prescribes Protocol that may be identified by one or more protocol codes. A Procedure Step involves equipment (e.g., imaging Modality equipment, anesthesia equipment, surgical equipment, transportation equipment), human resources, consumable supplies, location, and time (e.g., start time, stop time, duration). While in the context of Imaging Service request the scheduling of a Procedure Step might include only a general designation of imaging Modality that could be satisfied by multiple pieces of the same equipment type, the performance of one instance of a Procedure Step involves one and only one piece of imaging Modality equipment.
4.5.6.5 IPC-5 Modality (CWE) 01661
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The type of equipment requested to acquire data during performance of a Procedure Step. The acquired data will be used to create the images for the Imaging Study corresponding to the Requested Procedure.
This field is a case of the CE data type. Refer to External Table 0910 - Acquisition Modality in Chapter 2C, Code Tables, for valid values, and to DICOM Standard Part 3 for further details on DICOM Attribute (0008,0060) that conveys information identical to component one of this field.
A limit of sixteen (16) characters for the first component is required to allow compatibility with DICOM. The third component of this field, if present, shall have the value of "DCM" (see HL7 Table 0396 - Coding Systems in Chapter 2C, Code Tables).
4.5.6.6 IPC-6 Protocol Code (CWE) 01662
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: One or more coded entries identifying the protocol according to which the Scheduled Procedure Step shall be performed. Protocol Code(s) may identify particular equipment settings as well as operator's manipulations.
A Protocol is a specification of actions prescribed by a Procedure Plan to perform a specific Procedure Step. A Scheduled Procedure Step contains only one Protocol that may be conveyed by one or more Protocol Codes. Typically, the code or codes identifying Protocol instance would be selected from a catalog of protocols established locally or provided by equipment manufacturers or professional organizations. Multiple Protocols may not exist in one Scheduled Procedure Step. See DICOM Standard Part 3 for further details on DICOM Attribute (0040,0008) that conveys information identical to components one through three of this field.
A limit of sixteen (16) characters for the first component and sixty-four (64) characters for the second component is required to allow compatibility with DICOM.
4.5.6.7 IPC-7 Scheduled Station Name (EI) 01663
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field identifies the instance of the modality resource being requested for the performance of a particular Scheduled Procedure Step. It is a case of the Entity Identifier data type (section 2.A.28). The first component of this field is a string that identifies the particular piece of equipment. A limit of sixteen (16) characters is required to allow compatibility with DICOM. See DICOM Standard Part 3 for further details on DICOM Attribute (0040,0010) that conveys information identical to the component one of this field.
The second through fourth components identify the organization, in the form of the HD data type (see section 2.A.36, "HD - hierarchic designator").
If the Scheduled Procedure Step is to be performed by an unspecified member of a pool of resources, this field shall be empty and IPC-8 Scheduled Procedure Step Location is used to identify the site-specific resource pool. See section 4.5.6.8, "IPC-8 Scheduled Procedure Step Location (CWE) 01664," for explanation of the resource pool.
4.5.6.8 IPC-8 Scheduled Procedure Step Location (CWE) 01664
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field specifies a locally defined physical location of the modality resource being requested for performance of particular Scheduled Procedure Step. Although location is usually defined geographically (such as identification of a campus, building, floor, etc.) it may be used for identification of a pool of equipment (resources) formed by any other means. Values for the field shall be drawn from a locally defined coding scheme.
For example, the pool may be defined as a set of three CT scanners belonging to an imaging center within a hospital. Two of these scanners may also be grouped into another pool based on their location at a building A, whereas the third scanner may be in a pool by itself due to its location in a building B.
If this field contains more than one location code, the equipment may be drawn from several resource pools.
If this field is empty and the fields IPC-7 and IPC-9 are also empty, it is assumed that a particular Procedure Step may be performed by any instance of equipment of a particular type within an organization.
See DICOM Standard Part 3 for further details on DICOM Attribute (0040,0011) that conveys information identical to component one of this field. A limit of sixteen (16) characters for the first component is required to allow compatibility with DICOM.
4.5.6.9 IPC-9 Scheduled Station AE Title (ST) 01665
Definition: This field contains the Application Entity Title of the modality resource being requested for performance of a particular Scheduled Procedure Step. Application Entity Title is the identifier that identifies an instance of DICOM-compatible equipment for the purpose of addressing during communication. See DICOM Standard, Part 3 for further details on the DICOM Attribute (0040,0001) that conveys equivalent information. A limit of sixteen (16) characters is required to allow compatibility with DICOM.
If the Scheduled Procedure Step is to be performed by an unspecified member of a pool of resources, this field shall be empty and IPC-8 Scheduled Procedure Step Location is used to identify the site-specific resource pool. See section 4.5.6.8 for explanation of the resource pool.
4.6 GENERAL MESSAGE EXAMPLES
The purpose of this section is to show how certain specific situations would be handled using the order entry protocol. The ellipses represent uncompleted details. The symbol // precedes comments for clarification.
4.6.1 An order replaced by three orders
Suppose that an application called "PC" is sending an order to the EKG application for three EKGs to be done on successive days.
The order might be placed as follows:
ORM message:
MSH|...<cr>
PID|...<cr>
ORC|NW|A226677^PC||946281^PC||N|3^QAM||200601121132|444-44-4444^HIPPOCRATES^HAROLD^^^^MD|||4EAST|...<cr>
// EKG order
OBR|1|||8601-7^EKG IMPRESSION^LN||||||||||||222-33-4444^PUMP^PATRICK^^^^MD|||||||||||3^QAM|...<cr>
BLG|...<cr>
ORC|NW|...<cr>
// Another order yet others may follow
There is a group number first component indicating that an order group is being created.
Responses: Because the EKG application must turn the single order above into three orders for three separate EKGs (services), the results of each will be reported under its own OBR segment. Several response levels are possible depending on the Response Flag:
-
If the Response Flag is N (as it is), then the filler EKG application only responds "I got the order."
MSH|...<cr>
MSA|...<cr>
The only implication of this response is that the order was received.
If the Response Flag had been E, then the response would have been the same, but its implication would have been that the EKG application had processed all the orders and they were acceptable.
-
If the Response Flag were R, then the filler EKG application must communicate to the PC the fact of the creation of child orders, but with no details:
MSH|...<cr>
MSA|...<cr>
ORC|PA|A226677^PC|89-458^EKG|946281^PC<cr>
ORC|CH|A226677^PC|89-551^EKG|946281...<cr> // 1ST child ORC.
ORC|CH|A226677^PC|89-552^EKG|946281...<cr> // 2ND child ORC.
ORC|CH|A226677^PC|89-553^EKG|946281...<cr> // 3RD child ORC.
... // Other parts of follow.
What has been said here is "Your A226767 has spun out three children named 89-551, 89-552, and 89-553." Notice that the placer order numbers are identical in the children's ORCs.
-
If the Response Flag were D, then the filler EKG application must communicate to the PC application the fact of the replacement and also the exact replacement order segments:
MSH|...<cr>
MSA|...<cr>
ORC|PA|A226677^PC|89-458^EKG<cr>
ORC|CH|A226677^PC|89-551^EKG|946281^PC|SC|||A226677&PC^89-458&EKG|
... ^^^^198901130500^...<cr>
// 1ST child ORC
OBR|1||89-551^EKG|8601-7^EKG IMPRESSION^LN|...<cr>
// 1ST child OBR
ORC|CH|A226677^PC|89-522^EKG|946281^PC|SC|||A226677&PC^89-458&EKG|
... ^^^^198901140500^...<cr>
// 2ND child ORC
OBR|2||89-552^EKG|8601-7^EKG IMPRESSION^LN|...<cr>
// 2ND child OBR
ORC|CH|A226677^PC|89-553^EKG|946281^PC|SC|||A226677&PC^89-458&EKG|
...^^^^198901150500^...<cr>
// 3RD child ORC
OBR|3||89-553^EKG|8601-7^EKG IMPRESSION^LN|... <cr>
// 3RD child OBR
// Other parts might follow
Here the actual OBR segments have been added.
The status of the child orders is being reported as SC (scheduled).
ORC-7-quantity/timing shows that the EKGs are requested after 0500 on successive days.
4.6.2 Ordering non-medical services
The patient requests hospital specific services for a certain period of time. This can be a phone, fax, or TV in the room, or the delivery of a newspaper every day. Another example may be the use of specialized chip cards that give access to hospital specific services. Typically, a request for these services is made at the time of admission. Another example may be the printing of a form (e.g., the receipt for a payment). In case of using phones it might be a detailed list of calls for a patient or for a special extension.
To support these scenarios, the following fields are used to communicate the appropriate message:
|
Segment/Field
|
Definition
|
|
ORC-1
|
Order Control
|
|
ORC-2
|
Placer Order Number
|
|
ORC-5
|
Order Status
|
|
TQ1-7
|
Start Date/Time
|
|
TQ1-8
|
End Date/Time
|
|
ORC-16
|
Order Control Code Reason
|
|
ORC-25
|
Order Status Modifier
|
|
OBR-4
|
Universal Service ID
|
|
OBX-5
|
Observation Value
|
|
FT1-17
|
Fee Schedule
|
|
FT1-11
|
Transaction amount - extended
|
|
BLG
|
Billing segment
|
-
ORC-1, ORC-2, OBR-4, OBX-5 These services can be started, discontinued, canceled, locked, etc., according to the ORC-1 Order control code. The order is identified through ORC-2 Placer order number. The service itself is specified in the field OBR-4 Universal service ID. User defined codes are used to identify the specific services. The identification of the object of the service, e.g., phone number or card number, is done using the OBX-5 Observation value. The ORC-25 Order Status Modifier is used to refine the status of the universal service ID. For example, in the case of issuing chip cards, these fields would be valued as follows:
|
ORC-1
|
OBR-4 (in textual form)
|
ORC-16.1 Code
|
Description
|
|
NW
|
chip card
|
|
Issue a chip card the first time
|
|
XO
|
chip card
|
defective
|
Change the previous order. Issue a new chip card for a defective one.
|
|
XO
|
chip card
|
lost
|
Change the previous order. Issue a new chip card for a defective one.
|
|
DC
|
Return chip card
|
|
Cancel the chip card order
|
|
DC
|
Return chip card
|
lost
|
Cancel the chip card order because lost.
|
|
DC
|
Return chip card
|
defective
|
Cancel the chip card order because defective.
|
Use of different universal service IDs allows for the ability to charge an additional fee.
-
TQ1-7/8 The field TQ1 Quantity/timing describes time periods during which the requested service is valid. The components 4 and 5 denote the start and end date/time.
-
ORC-5 In this field information on the status of the service can be transmitted. This field can be used in particular in response to a query message.
-
ORC-25 This field allows for refining the status of the requested universal service, e.g., to change an order for a chip card in order to distribute a new card for a lost one.
-
BLG-1,2,3 These fields indicate to the financial system that charges are to be invoiced for this service.
-
FT1-17 In some cases it is necessary that the placer defines a special tariff the filler has to use for computing the final balance.
-
FT1-11 In combination with the tariff the patient can prepay the ordered service. This may be helpful when the patient uses services provided by the hospital in order to use the service from the beginning. FT1-6 must be valued at "PY". If no amount is prepaid a limit can be established according to a special tariff. This depends on the setup of the filling system. In such a case the hospital grants a credit to the patient.
Phone Number Assignment
In case the patient requests a bedside phone and the number of this phone is assigned to that patient personally, a number of messages are transmitted. The objective is to connect a phone number to a patient and a room.
The update of the location master file depends on the setup of the private branch exchange system (PABX):
-
Variable Numbering System On admission the patient is assigned his or her personal call number, which he or she retains throughout that patient's stay, including if the patient is transferred. The patient can always be reached under the same call number. To understand the mechanism for M05 events it is important to know that two different sets of phone numbers exist: one is a pool to be used when querying for a phone number for a patient; the other one is used for temporary assignments when no patient is lying in the bed (i.e., the bed is free).
-
Fixed Numbering System On admission the system issues the patient with a telephone and/or TV authorization. This authorization key must be entered into the phone to activate it. No M05 messages are necessary if a fixed numbering system is used: Each telephone connection is assigned a permanent call number when the system is set up.
When the patient is admitted, an ADT^A01 message is sent to create a patient record in the phone number assigning application. Typically, the patient ID (PID-3), patient location (PV1-3), and visit number (PV1-19) are at least required. This message is acknowledged accordingly with an ACK. Then, the order for the phone number to the phone number assigning application is placed with the ORM^O01 message where the essential fields are ORC-1 = "NW", ORC-2 = <placer order number>, and OBR-4 = "Phone".
The ORR^O02 message is used to acknowledge the order and communicate the filler order number and order status. Then, when the phone number is available, an ORU^R01 message is used to communicate the phone number using OBX-5 for the phone number.
Any status changes to the order are communicated with the ORM^O01 message where ORC-1 = "SC", ORC-2 = <placer order number>, ORC-3 = <filler order number>, ORC-5 = <order status>, OBR-4 = "Phone", and OBX-5 = <Phone Number of Patient>. The status change is acknowledged with the ORR^O02 message.
Next, the location master files are updated. The phone number assigning application may send a MFN^M05 message to have the location master file reflect the phone number assignment as well. The fields on the message are valued as follows:
After processing the order: MFI-1 = "LOC", MFI-3 = "UPD", MFI-5 = <effective date/time>, MFE-1 = "MUP", LOC-1 = <patient location>, LOC-3 = "B" (bed), LOC-6 = <Phone Number of Patient>. This message is acknowledged using the MFK^M05 message.
Transfer a patient (A02)
If a patient keeps the same phone number during the whole visit the assigned phone number must be mapped to a different phone outlet whenever a patient is transferred to a new location. In that case, the ADT^A02 message is sent to the phone number assigning application. That application not only acknowledges the message, but also sends an ORM^O01 message with ORC-1 = "SC" and the other fields the same as described in the Phone Number Assignment section. Additionally, it sends a MFN^M05 message to change the location master file accordingly for the old location and another MFN^M05 to synchronize the phones for the new location.
Leave of absence (A21/A22)
When the patient leaves the hospital or the bed is vacated for a significant amount of time, the phone needs to be de-activated and re-activated appropriately. The same ORM^O01 and MFN^M05 messages are used as described above following the ADT^A21 and ADT^22 messages.
Patient makes calls or (de-)activates his phone.
The patient can use the phone whenever he wants to. This implies that his balance does not exceed the limit. Otherwise the phone is deactivated automatically. Furthermore the patient can activate or deactivate the phone by entering the authorization key for his own. In these scenarios the phone number assigning application sends and ORM^O01 message with ORC-1 = "OD" and the appropriate order status. The status update is necessary to provide a call switching system with the actual information.
Discharge a patient (A03)
When the patient is discharged, the ADT^A03 message is sent to indicate a discharge. The phone number assigning application sends an ORM^O01 message with a change of status to indicate completion of the order, as well as an MFN^M05 message to synchronize the location master file.
After discharging a patient his final charges must be billed. Using the query P04 returns the data in a display oriented format which can be used for printing. Alternatively a print request can be used. The billing system issues a QRY^P04 message where the fields are valued as follows: QRD-2 = "R" (record oriented format), QRD-3 = "I" (immediate response), QRD-8.1 = <Patient ID>, QRF-2 = <start date/time>, and QRF-3 = <end date/time>. The phone number assigning applications responds with a DSR^P04 message with the data in DSP-3.
Note: The original mode query, including QRD and QRF segments were retained for backward compatibility only as of v 2.4. The reader is therefore referred to chapter 5, section 5.4, for the current query/response message structure.
Phone Call Queries (Z73)
The new query modes using a query by parameter query with a virtual table response allows for obtaining call information from the phone system to be used for charging. The query can be for accumulated data or detailed data. Both requests use this conformance statement:
QPD Input Parameter Specification:
|
Field Seq. (Query ID=Z73)
|
Name
|
Key/ Search
|
Sort
|
LEN
|
TYPE
|
Opt
|
Rep
|
Match Op
|
TBL
|
Segment Field Name
|
Service Identifier Code
|
ElementName
|
|
1
|
Patient ID
|
K
|
Y
|
80
|
CX
|
R
|
|
=
|
|
PID.3
|
|
PID.3 Patient ID
|
|
2
|
Date Range
|
|
|
53
|
DR
|
O
|
|
contains=
|
|
|
|
|
|
3
|
Detailed
|
|
|
2
|
ID
|
O
|
|
=
|
0136 Yes/No
|
|
|
|
Input Parameter Field Description and Commentary:
|
Field
|
Component
|
DT
|
Description
|
|
Patient ID
|
|
CX
|
Components: ^ ^ ^ ^ ^
|
|
|
|
This field contains a patient identification code to identify the requested person.
|
|
|
|
If this field is not valued, no values for this field are considered to be a match.
|
|
Date Range
|
|
DR
|
This field specifies the range of time, the requested records should match.
|
|
|
|
If this field is not valued, all values for this field are considered to be a match.
|
|
Detailed
|
|
ID
|
This field specifies whether the output should be detailed. (no cumulative records).
|
|
|
|
If this field is not valued, a detailed result is returned.
|
|
|
|
When Detailed=Y is requested, one record for each call is returned. Each detailed record will contain columns 1, 2, 3, 4, 5, 7, 8, and 9 (Providor, Region, Extension, Destination, Date/Time, Duration, Units, Amount) for each call.
|
|
|
|
When detailed=N, the query is for accumulated data. In this case, one row record per extension is returned.
|
|
|
|
Each row will return columns 1, 2, 6, 7, 8, and 9 (Provider, Region, Quantity, Units, Amount) from the output virtual table.
|
Response Grammar:
RTB^Z74^RTB_Z74: Personnel Information Message
|
|
|
|
MSH
|
Message Header
|
|
2.15.9
|
DB |
|
MSA
|
Message Acknowledgement
|
|
2.15.8
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2.15.5
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2.15.12
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
QAK
|
Query Acknowledgement
|
|
5.5.2
|
DB |
|
QPD
|
Query Parameter Definition
|
|
5.5.4
|
DB |
|
[
|
--- ROW_DEFINITION begin
|
|
|
|
|
RDF
|
Table Row Definition Segment
|
|
5.5.7
|
DB |
|
[
{
RDT
}
]
|
Table Row Data Segment
|
|
5.5.8
|
DB |
|
]
|
--- ROW_DEFINITION end
|
|
|
|
|
[
DSC
]
|
Continuation Pointer
|
|
2.15.4
|
DB |
Virtual Table:
|
ColName (Z74)
|
Key/
Search
|
Sort
|
LEN
|
TYPE
|
Opt
|
Rep
|
Match Op
|
TBL
|
Segment Field Name
|
LOINC or HL7 code
|
ElementName
|
|
Provider
|
|
|
40
|
ST
|
R
|
|
|
|
|
|
|
|
Region
|
|
|
40
|
ST
|
R
|
|
|
|
|
|
|
|
Extension
|
|
|
250
|
XTN
|
O
|
|
|
|
|
|
|
|
Destination number
|
|
|
250
|
XTN
|
O
|
|
|
|
|
|
|
|
Date/Time
|
|
Y
|
24
|
DTM
|
O
|
|
|
|
|
|
|
|
Quantity
|
|
|
4
|
NM
|
O
|
|
|
|
|
|
|
|
Duration
|
|
|
4
|
NM
|
O
|
|
|
|
|
|
|
|
Units
|
|
|
4
|
NM
|
O
|
|
|
|
|
|
|
|
Amount
|
|
|
8
|
MO
|
O
|
|
|
|
|
|
|
4.6.2.0
4.6.2.1 Examples
Example 1:
Query the accumulated list for patient 12345 from 3/2/00 till 3/3/00. Transfer the first 20 records.
Query:
MSH|^&~\|PCR|Gen Hosp|Pharm||20000303201400-0800||QBP^Z73^QBP_Z73|9901|P|2.8|
QPD|Z89^Query Phone Calls^HL70471|Q010|12345|2000030100000^20000302235959|Y
RCP|I|20^RD|
Answer:
MSH|^&~\|Pharm|Gen Hosp|PCR||20000303201430-0800||RTB^Z74^RTB_Z74|8858|P|2.8|
MSA|AA|9901|
QAK|Q010|OK|Z89^Query Phone Calls^HL70471|4
QPD|Z89^Query Phone Calls^HL70471|Q010|12345|2000030100000^20000302235959|Y|
RDF|9|Provider^ST^20|Region^ST^40|Extension^XTN^40|Destination^XTN^40|Date/Time^DTM^24|Quantity^NM^4|Duration^NM^4|Units^NM^4|Amount^MO^8|
RDT|DTAG|CITY||||5|20|3|3.25|
RDT|DTAG|R50||||1|10|2|1.00|
RDT|DTAG|R200||||0|0|0|0|
RDT|DTAG|NAT||||0|0|0|0|
RDT|DTAG|INT||||0|0|0|0|
Example 2:
Query the detailed information for patient 12345 from 3/1/06 till 3/3/06. Transfer the first 10 records.
Query:
MSH|^&~\|PCR|Gen Hosp|Pharm||200611201400-0800||QBP^Z73^QBP_Z73|ACK9901|P|2.8|
QPD|Z89^Query Phone Calls^HL70471|Q010|12345|2006030100000^20060302235959|Y|
RCP|I|10^RD|
Answer:
MSH|^&~\|Pharm|Gen Hosp|PCR||200611201401-0800||RTB^Z74^RTB_Z74|8858|P|2.8|
MSA|AA|8858 QAK|Q010|OK|Z89^Query Phone Calls^HL70471|4
QPD|Z89^Query Phone Calls^HL70471|Q010|12345|2006030100000^20060302235959|Y|
RDF|9|Provider^ST^20|Region^ST^40|Extension^XTN^40|Destination^XTN^40|Date/Time^DTM^24|Quantity^NM^4|Duration^NM^4|Units^NM^4|Amount^MO^8|
RDT|DTAG|CITY|12345|555-1234|200603021715||20|12|2.25|
RDT|DTAG|CITY|12345|555-4569|200603011252||21|3|0.48|
Requesting a Chip card
In case the hospital provides additional services that can be accessed through chip cards, this card has to be issued to the patient. At the end of the visit this chip card is returned. Distributing a chip card to a patient is a service which must be ordered from the chip card dispensing system, too. When discharging the patient the service (= order) is complete.
The messages are essentially the same as for issuing a phone number. The filler for the chip card order is a chip card dispensing application and instead of returning a phone number, it returns a chip card number. The following scenarios have slight variations.
New Chip Card requested due to, e.g., loss
When a card is lost, or a new chip card must be requested, an additional fee can be communicated by including the FT1 segment in the ORM^O01 message and valuing FT1-11 = <additional fee>.
Request a new Chip card for a defective one
Sometimes a chip card is defective. Then the patient needs a new one. This situation requires an order using the XO control code in the ORM^O01 message. The chip card dispensing system returns the new chip card number using the ORU^RO1. The ORC-16-Order Control Code Reason is used to clarify the request.
Return a chip card
When the patient returns the chip card, a discontinue message is send with ORC-1 = "DC". This message is acknowledged accordingly by the chip card dispensing system.
Printing a form
When form needs printing, the ORM^O01 could also be used. The OBR segment would contain the print form service and the OBX would contain the specific print form. A notification when completing the printing is feasible as well using the ORM^O01 with a status update associated to the appropriate placer/filler order number.
4.7 DIET TRIGGER EVENTS & MESSAGE DEFINITIONS
A diet office needs to receive specific information, the most important being the diet order itself. Diet restrictions (often called diet codes) are the basic building blocks of a diet order. The diet order segments may be sent as part of the ORM and ORR message structure to support backwards compatibility, or may be sent as part of the following dedicated message structures.
4.7.1 OMD - Dietary Order (Event O03)
OMD^O03^OMD_O03: Dietary Order
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER_DIET begin
|
|
|
|
|
ORC
|
Common Order Segment
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING_DIET begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_DIET end
|
|
|
|
|
[
|
--- DIET begin
|
|
|
|
|
{
ODS
}
|
Dietary Orders, Suppl., Prefer.
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for ODS)
|
|
2
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Results
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for OBX)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
]
|
--- DIET end
|
|
|
|
|
}
|
--- ORDER_DIET end
|
|
|
|
|
[{
|
--- ORDER_TRAY begin
|
|
|
|
|
ORC
|
Common Order Segment
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING_TRAY begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_TRAY end
|
|
|
|
|
{
ODT
}
|
Diet Tray Instructions
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for ODT)
|
|
2
|
DB |
|
}]
|
--- ORDER_TRAY end
|
|
|
|
4.7.2 ORD - dietary order acknowledgment (Event O04)
ORD^O04^ORD_O04: Dietary Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for MSA)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER_DIET begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING_DIET begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_DIET end
|
|
|
|
|
[
{
ODS
}
]
|
Dietary Orders, Supplements, and Preferences
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for ODS)
|
|
2
|
DB |
|
}
|
--- ORDER_DIET end
|
|
|
|
|
[{
|
--- ORDER_TRAY begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING_TRAY begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING_TRAY end
|
|
|
|
|
[
{
ODT
}
]
|
Diet Tray Instructions
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for ODT)
|
|
2
|
DB |
|
}]
|
--- ORDER_TRAY end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.8 DIET SEGMENTS
4.8.1 ODS - dietary orders, supplements, and preferences segment
The ORC sequence items of interest to ODS are ORC-1-order control, ORC-2-placer order number, ORC-3-filler order number, TQ1-7/8-quantity/timing, ORC-9-date/time of transaction, ORC-10-entered by, and ORC-11-verified by. For ORC-1-order control, the values may be New (NW), Cancel (CA), Discontinue Order Request (DC), Change (XO), Hold Order Request (HD), and Release Previous Hold (RL). The HD and RL codes could stop service for a specified length of time. TQ1-quantity/timing should be used to specify whether an order is continuous or for one service period only. It is also useful for supplements which are part of a diet but only delivered, say, every day at night.
Example:
|1^QPM^^20010415|.
HL7 Attribute Table - ODS - Dietary Orders, Supplements, and Preferences
|
SEQ
|
LEN
|
C.LEN
|
DT
|
OPT
|
RP/ #
|
TBL #
|
ITEM #
|
ELEMENT NAME
|
DB Ref. |
|
1
|
1..1
|
|
ID
|
R
|
|
0159
|
00269
|
Type
|
DB |
|
2
|
|
|
CWE
|
O
|
Y/10
|
9999
|
00270
|
Service Period
|
DB |
|
3
|
|
|
CWE
|
R
|
Y/20
|
9999
|
00271
|
Diet, Supplement, or Preference Code
|
DB |
|
4
|
|
80#
|
ST
|
O
|
Y/2
|
|
00272
|
Text Instruction
|
DB |
4.8.1.0 ODS field definitions
4.8.1.1 ODS-1 Type (ID) 00269
Definition: This field specifies type of diet. Refer To HL7 Table 0159 - Diet Code Specification Type in Chapter 2C, Code Tables, for valid entries.
4.8.1.2 ODS-2 Service Period (CWE) 00270
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: When blank, the modifier applies to all service periods. Diet orders, for example, typically apply to all service periods. This field usually specifies supplements. This field allows you to designate a modification for one or more of the service periods during a day by combining service specifications as needed. The service periods will be local CEs, normally numbers. Suggested are:
service 1
is
breakfast
service 2
is
mid-morning snack
service 3
is
lunch
service 4
is
mid-afternoon snack
service 5
is
dinner
service 6
is
bedtime snack
Ex: |1~5| means service 1 and service 5, whatever these are locally defined to be.
4.8.1.3 ODS-3 Diet, Supplement, or Preference Code (CWE) 00271
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the identifier of the ordered item for a patient; it is equivalent to OBR-4-universal service ID in function. Since ODS is a repeating segment, multiple entities get multiple segments. Example:
|^REG|, |023^^99FD6|, |^NOLACT|, |^TUBEFD|, and |011^HIPRO100^99FD1~123^LOFAT20^99FD1|
In the case where this segment requests a diet supplement, i.e., ODS-1-type = S, this attribute specifies a particular item or class of items. If institutional codes for patient food preferences (P) have been codified, they are also expressed as coded segments; otherwise, the information is passed as a text string in the fourth component of the ODS segment, described below.
4.8.1.4 ODS-4 Text Instruction (ST) 00272
Definition: This field defines the specific instructions for dietary. These instructions may address specific patient needs, such as isolation. This field provides the ordering provider's dietary instructions as free text. It can represent the full dietary instruction or indicate supplemental information.
4.8.2 ODT - diet tray instructions segment
This segment addresses tray instructions. These are independent of diet codes, supplements, and preferences and therefore get separate order numbers.
HL7 Attribute Table - ODT - Diet Tray Instructions
|
SEQ
|
LEN
|
C.LEN
|
DT
|
OPT
|
RP/ #
|
TBL #
|
ITEM #
|
ELEMENT NAME
|
DB Ref. |
|
1
|
|
|
CWE
|
R
|
|
0160
|
00273
|
Tray Type
|
DB |
|
2
|
|
|
CWE
|
O
|
Y/10
|
9999
|
00270
|
Service Period
|
DB |
|
3
|
|
80#
|
ST
|
O
|
|
|
00272
|
Text Instruction
|
DB |
4.8.2.0 ODT field definitions
4.8.2.1 ODT-1 Tray Type (CWE) 00273
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field defines the type of dietary tray. Refer To HL7 Table 0160 - Tray Type in Chapter 2C, Code Tables, for valid entries.
Tray specifications are useful for early and late tray delivery in cases where a patient undergoes a procedure during normal feeding times. Tray specifications can also be used for guest trays, no trays, and messages. The value MSG means the ODT segment does not specify the type of tray but provides additional information about an existing tray. This information is found in ODT-3-text instruction.
4.8.2.2 ODT-2 Service period (CWE) 00270
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: When blank, the modifier applies to all service periods. This field allows you to designate one or more of the feeding periods during a day by combining the codes as needed. It can also combine with quantity/timing to give such information as which service period the order belongs with. This field is identical in meaning with ODS-2-service period. See section 4.8.1.2, "ODS-2 Service Period (CWE) 00270," for further details.
4.8.2.3 ODT-3 Text Instruction (ST) 00272
Definition: This field defines instructions associated with the tray. Example:
|PLASTIC SILVERWARE|.
4.9 DIET MESSAGE EXAMPLES
4.9.1 Typical progression of orders for a surgery patient
First order:
MSH|...<cr>
PID|...<cr>
ORC|NW|1235^NURS|||||^^^199108021700||200608021200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||321^DB15^99DO3|...<cr>
ODS|D||322^NA2GM^99DO3|<cr>
Hold first order:
MSH|...<cr>
PID|...<cr>
ORC|HD|1235^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
NPO order with guest tray:
MSH|...<cr>
PID|...<cr>
ORC|NW|1236^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||323^NPO^99DO3|...<cr>
ORC|NW|1244^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|GUEST^Guest tray^HL70160|5^^99CBD|...<cr>
Clear liquid with guest tray:
MSH|...<cr>
PID|...<cr>
ORC|DC|1236^NURS|||||^^^200608041700||200608041200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ORC|NW|1237^NURS|||||^^^200608041700||200608041200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||321^DB15^99DO3|...<cr>
ODS|D||322^NA2GM^99DO3|...<cr>
ODS|D||324^CLRLIQ^99DO3|...<cr>
ORC|NW|1245^NURS|||||^^^200608041700||200608041200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|GUEST^Guest tray^HL70160|5^^99CBD|...<cr>
Full liquid with guest tray:
MSH|...<cr>
PID|...<cr>
ORC|DC|1237^NURS|||||^^^200608051700||200608051200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ORC|NW|1238^NURS|||||^^^200608051700||200608051200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||321^DB15^99DO3|...<cr>
ODS|D||322^NA2GM^99DO3|...<cr>
ODS|D||325^FULLIQ^99DO3|...<cr>
ORC|NW|1246^NURS|||||^^^200608051700||200608051200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|GUEST^Guest tray^HL70160|3^^99CBD|...<cr>
Release hold on previous order and give discharge message:
MSH|...<cr>
PID|...<cr>
ORC|DC|1238^NURS|||||^^^200608061700||200608061200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ORC|RL|1235^NURS|||||^^^200608061700||200608061200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ORC|NW|1247^NURS|||||^^^200608061700||200608061200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|MSG^Tray message only^HL70160|5^^99CBD|You Will Be Leaving Tomorrow|...<cr>
4.9.2 Complex order
Basic diet: high protein, low fat. Supplements are ice cream at service period 4 and a half ham sandwich at service period 6. There are also tray orders for early service period 1, late service period 3, and guest tray at dinner.
MSH|...<cr>
PID|...<cr>
ORC|NW|1234^NURS|||||^^^200608021700||200608021200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||011^HIPRO100^99FD1|...<cr>
ODS|D||123^LOFAT20^99FD1|...<cr>
ODS|S|4|119^ICE CREAM^99FD8|...<cr>
ODS|S|6|320^1/2 HAM SANDWICH^99FD8|...<cr>
ORC|NW|1244^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|EARLY^Early tray^HL70160|1^^99CBD|...<cr>
ORC|NW|1245^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|LATE^Late tray^HL70160|3^^99CBD|...<cr>
ORC|NW|1246^NURS|||||^^^200608031700||200608031200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODT|GUEST^Guest tray^HL70160|5^DINNER^99CBD|...<cr>
4.9.3 Tube feeding
This order specifies Similac with MCT oil and polycose additives.
MSH|...<cr>
PID|...<cr>
ORC|NW|1232^NURS|||||60^Q3H^^200608021700||200608021200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||010^SIMILAC^99DO1|...<cr>
ODS|D||011^MCT^99DO1|...<cr>
ODS|D||012^POLYCOSE^99DO1|...<cr>
4.9.4 Patient preference
This order specifies that the patient is a vegetarian.
MSH|...<cr>
PID|...<cr>
ORC|NW|1232^NURS|||||60^Q3H^^200608021700||200608021200|333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|...<cr>
ODS|D||123^LOFAT20^99FD1|...<cr>
ODS|S|4|119^ICE CREAM^99FD8|...<cr>
ODS|P||^VEGETARIAN|...<cr>
4.10 SUPPLY TRIGGER EVENTS & MESSAGES
The Requisition Detail segment (RQD) is used for ordering medical, surgical, and patient care supplies. It is assumed that these supplies are managed by a materials management application, which contains a master list of all items the hospital uses.
There are basically two types of supplies, commonly referred to as stock and non-stock.
Stock supplies are, as the name suggests, stocked in the hospital in designated areas, such as the warehouse, Central Supply, Nursing floors, or Operating Room. When requisitioning stock supplies, the requesting application need only specify the information in the RQD segment. It is assumed that this is enough information for the application receiving to identify the item. If the sending application is not aware whether the supply is stock, it may optionally send an RQ1 along with the RQD. Typically in that case, the item is requested with a free text description.
Non-stock supplies are not stocked anywhere in the hospital and must be ordered from an industry distributor or manufacturer. When the requesting application knows that it is requisitioning non-stock supplies, it may also send an RQ1 segment with the RQD if at least one field in RQ1 is known to the sending application. This may be necessary in order for the receiving application to properly determine where to get these supplies. This depends on the sophistication of the database of the receiving application, which may contain a history of requisitions from the sending application.
4.10.1 OMS - stock requisition order message (event O05)
Stock requisition orders use the ORM where RQD is the detail segment for backward compatibility or can use the OMS and ORS messages described below.
OMS^O05^OMS_O05: Stock Requisition Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
15
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
RQD
|
Requisition Detail
|
|
4
|
DB |
|
[
RQ1
]
|
Requisition Detail-1
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for RQD)
|
|
2
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for OBX)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.10.2 ORS - stock requisition order acknowledgment message (event O06)
ORS^O06^ORS_O06: Stock Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
RQD
|
Requisition Detail
|
|
4
|
DB |
|
[
RQ1
]
|
Requisition Detail-1
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for RQD)
|
|
2
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.10.3 OMN - non-stock requisition order message (event O07)
Non-stock requisitions can use the ORM message with the RQD and RQ1 segments as the detail segment, or use the OMN and ORN messages described below:
OMN^O07^OMN_O07: Nonstock Requisition Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
RQD
|
Requisition Detail
|
|
4
|
DB |
|
[
RQ1
]
|
Requisition Detail-1
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for RQD)
|
|
2
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for OBX)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[
BLG
]
|
Billing Segment
|
|
4
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.10.4 ORN - non-stock requisition order acknowledgment message (event O08)
ORN^O08^ORN_O08: General Order Acknowledgment Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
RQD
|
Requisition Detail
|
|
4
|
DB |
|
[
RQ1
]
|
Requisition Detail-1
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for RQD)
|
|
2
|
DB |
|
}
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.11 SUPPLY SEGMENTS
4.11.1 RQD - Requisition Detail Segment
RQD contains the detail for each requisitioned item. See assumptions above.
HL7 Attribute Table - RQD - Requisition Detail
4.11.1.0 RQD field definitions
4.11.1.1 RQD-1 Requisition Line Number (SI) 00275
Definition: This field contains the number that identifies this line in the requisition.
4.11.1.2 RQD-2 Item Code - Internal (CWE) 00276
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identifier and description that uniquely identify the item on the application sending the requisition. This field is conditional because at least one of the three fields - RQD-2-item code- internal, RQD-3-item code-external, or RQD-4-hospital item code - must be valued.
4.11.1.3 RQD-3 Item Code - External (CWE) 00277
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identifier and description that uniquely identify the item on the application receiving the requisition. This field is conditional because at least one of the three fields - RQD-2-item code-internal, RQD-3-item code-external or RQD-4-hospital item code - must be valued.
4.11.1.4 RQD-4 Hospital Item Code (CWE) 00278
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identifier and description that uniquely identify the item on all applications in the hospital. The identifier is usually controlled by the hospital financial application in the charge description master file. This field is conditional because at least one of the three fields - RQD-2-item code-internal, RQD-3-item code-external or RQD-4-hospital item code -- must be valued.
Note: At least one of the three fields 4.11.1.2 through 4.11.1.4 must be non-null.
4.11.1.5 RQD-5 Requisition Quantity (NM) 00279
Definition: This field contains the quantity requisitioned for this item.
4.11.1.6 RQD-6 Requisition Unit of Measure (CWE) 00280
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the unit of measure for this item.
4.11.1.7 RQD-7 Cost Center Account Number (CX) 00281
Components: <ID Number (ST)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Effective Date (DT)> ^ <Expiration Date (DT)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the general ledger cost center account number associated with a department that may issue or charge for this item. Refer to HL7 Table 0319 - Department Cost Center in Chapter 2C, Code Tables, for valid values.
4.11.1.8 RQD-8 Item Natural Account Code (CWE) 00282
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the accounting code that identifies this item in order to charge for this item. User-Defined Table 0320 - Item Natural Account Code in Chapter 2C, Code Tables, is used as the HL7 identifier for the user-defined table of values for this field.
4.11.1.9 RQD-9 Deliver to ID (CWE) 00283
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the unique identifier and descriptive name of the department/location where the item should be delivered.
4.11.1.10 RQD-10 Date Needed (DT) 00284
Definition: This field contains the date this item is required.
Note: Although none of the fields are required, one of the three identifying codes-RQD-2-item code-internal, RQD-3-item code-external, or RQD-4-hospital item code-must be specified in order for the receiving application to process the request.
It is left to the vendors to determine which will be used as the common link between the two applications. HL7 recommends using the RQD-4-Hospital Item Code.
Hospital accounting requires an identifier to charge a particular cost center or patient for a requisitioned supply. If the supply is for a patient, then this identifier comes from the PID segment; otherwise, from RQD-7-Dept. Cost Center and RQD-8-Item Natural Account Code must be used. It is recommended that the "final" cost center responsible for providing the supply to the patient be included, even when the patient ID is provided.
Hospital accounting applications use RQD-7-Dept. Cost Center concatenated with RQD-8-Item Natural Account Code in order to post this transaction to the General Ledger. This concatenated value should correspond to a valid entry in the accounting applications "Chart of Accounts."
4.11.2 RQ1 - Requisition Detail-1 Segment
RQ1 contains additional detail for each non-stock requisitioned item. This segment definition is paired with a preceding RQD segment.
HL7 Attribute Table - RQ1 - Requisition Detail-1
4.11.2.0 RQ1 field definitions
4.11.2.1 RQ1-1 Anticipated Price (ST) 00285
Definition: This field contains the reference price for the requisition unit of measure that is known to the requisition application. It may or may not be the actual cost of acquiring the item from a supplier. It is also not the price charged to the patient.
4.11.2.2 RQ1-2 Manufacturer Identifier (CWE) 00286
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the unique code that identifies the manufacturer on the application receiving the requisition. This field is conditional because either RQ1-2-manufacturer ID and RQ1-3-manufacturer's catalog or RQ1-4-vendor ID and RQ1-5-vendor catalog must be valued.
Refer to User-defined Table 0385 - Manufacturer identifier in Chapter 2C, Code Tables, for suggested values, or relevant external code sets may be used (e.g., HIBCC Manufacturers Labeler ID Code (LIC), UPC, NDC).
4.11.2.3 RQ1-3 Manufacturer's Catalog (ST) 00287
Definition: This field is the manufacturer's catalog number or code for this item. This field is conditional because either RQ1-2-manufacturer ID and RQ1-3-manufacturer's catalog or RQ1-4-vendor ID and RQ1-5-vendor catalog must be valued.
4.11.2.4 RQ1-4 Vendor ID (CWE) 00288
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field is the unique code that identifies the vendor on the application receiving the requisition. This field is conditional because either RQ1-2-manufacturer ID and RQ1-3-manufacturer's catalog or RQ1-4-vendor ID and RQ1-5-vendor catalog must be valued.
Because of this, it is recommended that each non-stock item have RQ1-2-manufacturers ID and RQ1-3-manufacturer's catalog, or RQ1-4-vendor ID and RQ1-5-vendor catalog. It is also possible that the requisitioning application will not know the identifier, as listed in the Manufacturer's or Vendor's catalog. In this case, it is important to include the name portion of this coded element field.
4.11.2.5 RQ1-5 Vendor Catalog (ST) 00289
Definition: This field is the vendor's catalog number, name, or code for this item. This field is conditional because either RQ1-2-manufacturer ID and RQ1-3-manufacturer's catalog or RQ1-4-vendor ID and RQ1-5-vendor catalog must be valued.
4.11.2.6 RQ1-6 Taxable (ID) 00290
Definition: This field indicates whether this item is subject to tax.
In general, non-stock requisitioned items will be printed by the receiving application and then processed by a human. In other words, the human will use the information to call the vendor or manufacturer to get pricing and other related purchasing information before placing the order with an outside vendor. Refer to HL7 Table 0136 -Yes/No Indicator as defined in Chapter 2C, Code Tables.
4.11.2.7 RQ1-7 Substitute Allowed (ID) 00291
Definition: This field indicates whether the ancillary department may substitute an equivalent version of the item(s) ordered. Refer to HL7 Table 0136 - Yes/No Indicator as defined in Chapter 2C, Code Tables.
4.12 SUPPLY MESSAGE EXAMPLES
4.12.1 Patient order
This example is a requisition from the ORSUPPLY application to the MMSUPPLY application for two items for patient Adam A. Everyman. One item is a stock item for an IV Solution and the second item is a nonstock implant manufactured by Detter. The requisition numbers used by the ORSUPPLY application are RQ101 & RQ102.
MSH|^~\&|ORSUPPLY|ORSYS|MMSUPPLY|MMSYS|20061105131523||OMS^O05^OMS_O05| ...<cr>
PID|... <cr>
ORC|NW|RQ101^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V|MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|1|1234^Solution, 2.25% Saline||S1786^Saline Solution|1|BT^Bottle|1234-5678||ORSUP^Main OR Supply Room|20061123|...<cr>
MSH|^~\&|ORSUPPLY|ORSYS|MMSUPPLY|MMSYS|19911105131523||OMN^O07^OMN_O07|...<cr>
PID|... <cr>
ORC|NW|RQ102^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304<cr>
RQD|1|23455^Implant, Special Hip||I45323^Implant|1|EA^ Each|1234-5678||ORSUP^Main OR Supply Room|20061123|...<cr>
RQ1|123.45|DET^Detter, Inc.|444456|DST^Local Distributors, Inc.|333-456|N|...<cr>
4.12.2 Replenish Supply Closet
This example is a requisition from the ORSUPPLY application to the MMSUPPLY application for five stock items to replenish a supply closet. The requisition numbers used by the ORSUPPLY application is RQ103 - RQ1037.
MSH|^~\&|ORSUPPLY|ORSYS|MMSUPPLY|MMSYS|20061105131523||OMS^O05^OMS_O05|...<cr>
ORC|NW|RQ103^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|1|1232^Solution, 1% Saline||S1784^Saline Solution|5|BT^Bottle|1234-5678||ORSUP^Main OR Supply Room|20061105|...<cr>
ORC|NW|RQ104^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|2|1231^Solution, 0.2% Saline||S1781^Saline Solution|2|BT^Bottle|1234-5678||ORSUP^Main OR Supply Room|20061105|...<cr>
ORC|NW|RQ105^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|3|2342^Suture, Black Silk||SU123^Suture|2|DZ^Dozen|1234-5678||ORSUP^Main OR Supply Room|20061105|...<cr>
ORC|NW|RQ106^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|4|2344^Suture, Black Silk 3-0||SU124^Suture|1|DZ^Dozen|1234-5678||ORSUP^Main OR Supply Room|20061105|...<cr>
ORC|NW|RQ107^ORSUPPLY||||N|||20061105130000||333-77-7777^COMRAD^CONNOR^C|999-99-9999^VERIFY^VIRGIL^V |MAINOR^2W|321-1234 X2304^^^^^^3211234^2304|...<cr>
RQD|5|4565^Bandage Pad, 4x4||B6345^Bandage Pad|3|BX^Box|1234-5678||ORSUP^Main OR Supply Room|20061105|...<cr>
4.13 TRANSFUSION SERVICE (BLOOD BANK) TRIGGER EVENTS & MESSAGES
4.13.1 Usage notes for transfusion service messages
Blood product order messages present the need for additional information that is not included in standard HL7 order messages. Order messages must contain accompanying details regarding the blood product component, such as special processing requirements (e.g., irradiation and leukoreduction), and the amount of the blood product to be administered. Additionally, specific relevant clinical information can be included to allow the prospective review of the appropriateness of the blood product order.
Blood product orders use the OMB message with the BPO segment for the detail segment and the acknowledgment message, ORB as described below.
OMB^O27^OMB_O27: Blood Product Order Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
[{
|
--- INSURANCE begin
|
|
|
|
|
IN1
|
Insurance
|
|
6
|
DB |
|
[
IN2
]
|
Insurance Additional Information
|
|
6
|
DB |
|
[
IN3
]
|
Insurance Additional Information, Certification
|
|
6
|
DB |
|
}]
|
--- INSURANCE end
|
|
|
|
|
[
GT1
]
|
Guarantor
|
|
6
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
|
3
|
DB |
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/QuantitÃ
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
BPO
|
Blood Product Order
|
|
4
|
DB |
|
[
SPM
]
|
Specimen
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Order)
|
|
2
|
DB |
|
[
{
DG1
}
]
|
Diagnosis
|
|
6
|
DB |
|
[{
|
--- OBSERVATION begin
|
|
|
|
|
OBX
|
Observation/Result
|
|
7
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Results)
|
|
2
|
DB |
|
}]
|
--- OBSERVATION end
|
|
|
|
|
[
{
FT1
}
]
|
Financial Transaction
|
|
6
|
DB |
|
[
BLG
]
|
Billing Segment
|
|
6
|
DB |
|
}
|
--- ORDER end
|
|
|
|
4.13.1.0 OMB use notes
The NTE segment(s) can be included in the OMB message in four places; in each place the NTE refers to the segment that it follows. In particular, the NTEs following the MSH refer only to the message header; the NTEs following the blood product order segment apply to the service defined by that ORC and blood product order segment.
The PID segment is required if and only if new orders are being entered and they are related to a particular patient. For non-patient-related orders the PID segment is never included.
The optional PV1 segment is present mainly to permit transmission of patient visit information such as current location with an order.
4.13.2 ORB - Blood Product Order Acknowledgment (Event O28)
ORB^O28^ORB_O28: Description
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Response Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
BPO
]
|
Blood Product Order
|
|
4
|
DB |
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- PATIENT end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.13.3 BPS - Blood Product Dispense Status Message (Event O29)
In the pre-transfusion processing of blood products, it is necessary for the transfusion service and the placer system to communicate information that is not included in the current HL7 order/observation model. Examples of pre-transfusion processing include performing a crossmatch test to ensure compatibility with the patient, or irradiation of the blood product due to a special transfusion requirement for the patient. The blood product dispense status messages need to contain additional information regarding the blood products requested, such as the Donation ID, product code, blood type, expiration date/time and current status of the blood product.
In the processing of commercial blood products, such as Rh Immune Globulin, Factor Concentrate, or Albumin Products, the status messages need to contain additional information, such as the lot number and manufacturer, expiration date and status of the commercial product.
Blood product dispense status messages use the BPS and BRP messages as described below.
BPS^O29^BPS_O29: Blood Product dispense status Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
BPO
|
Blood Product Order
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for BPO)
|
|
2
|
DB |
|
[{
|
--- PRODUCT begin
|
|
|
|
|
BPX
|
Blood Product Dispense Status
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for BPX)
|
|
2
|
DB |
|
}]
|
--- PRODUCT end
|
|
|
|
|
}
|
--- ORDER end
|
|
|
|
4.13.4 BRP - Blood Product Dispense Status Acknowledgment (Event O30)
BRP^O30^BRP_O30: Description
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Response Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
BPO
]
|
Blood Product Order
|
|
4
|
DB |
|
[
{
BPX
}
]
|
Blood Product dispense status
|
|
|
DB |
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- PATIENT end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.13.5 BTS - Blood Product Transfusion/Disposition Message (Event O31)
Blood product transfusion/disposition messages use the BTS and BRT messages as described below.
BTS^O31^BTS_O31: Blood Product Transfusion/Disposition Message
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Header)
|
|
2
|
DB |
|
[
|
--- PATIENT begin
|
|
|
|
|
PID
|
Patient Identification
|
|
3
|
DB |
|
[
PD1
]
|
Additional Demographics
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient)
|
|
7
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Patient ID)
|
|
2
|
DB |
|
[
|
--- PATIENT_VISIT begin
|
|
|
|
|
PV1
|
Patient Visit
|
|
3
|
DB |
|
[
PV2
]
|
Patient Visit - Additional Info
|
|
3
|
DB |
|
[
{
PRT
}
]
|
Participation (for Patient Visit)
|
|
7
|
DB |
|
]
|
--- PATIENT_VISIT end
|
|
|
|
|
]
|
--- PATIENT end
|
|
|
|
|
{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
BPO
|
Blood Product Order
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for BPO)
|
|
2
|
DB |
|
[{
|
--- PRODUCT_STATUS begin
|
|
|
|
|
BTX
|
Blood Product Transfusion/Disposition Status
|
|
4
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for BTX)
|
|
2
|
DB |
|
}]
|
--- PRODUCT_STATUS end
|
|
|
|
|
}
|
--- ORDER end
|
|
|
|
4.13.6 BRT - Blood Product Transfusion/Disposition Acknowledgment (Event O32)
BRT^O32^BRT_O32: Description
|
|
|
|
MSH
|
Message Header
|
|
2
|
DB |
|
MSA
|
Message Acknowledgment
|
|
2
|
DB |
|
[
{
ERR
}
]
|
Error
|
|
2
|
DB |
|
[
{
SFT
}
]
|
Software
|
|
2
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
2
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Response Header)
|
|
2
|
DB |
|
[
|
--- RESPONSE begin
|
|
|
|
|
[
PID
]
|
Patient Identification
|
|
3
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
[{
|
--- ORDER begin
|
|
|
|
|
ORC
|
Common Order
|
|
4
|
DB |
|
[
{
PRT
}
]
|
Participation
|
|
7
|
DB |
|
[{
|
--- TIMING begin
|
|
|
|
|
TQ1
|
Timing/Quantity
|
|
4
|
DB |
|
[
{
TQ2
}
]
|
Timing/Quantity Order Sequence
|
|
4
|
DB |
|
}]
|
--- TIMING end
|
|
|
|
|
[
BPO
]
|
Blood Product Order
|
|
4
|
DB |
|
[
{
BTX
}
]
|
Blood Product Transfusion/Disposition Status
|
|
4
|
DB |
|
}]
|
--- ORDER end
|
|
|
|
|
]
|
--- RESPONSE end
|
|
|
|
4.14 TRANSFUSION SERVICE (BLOOD BANK) SEGMENTS
4.14.1 BPO - Blood Product Order Segment
Blood product order messages require additional information that is not available in other standard HL7 order messages. Blood product order messages need to contain accompanying details regarding the blood product component, such as special processing requirements (e.g., irradiation and leukoreduction) and the amount of the blood product to be administered.
The following table presents various use cases surrounding blood product orders.
|
Universal Service ID [ISBT-128 Product Code]
|
Blood Product Processing Requirements
|
Quantity
|
Blood Product Amount
|
Units
|
|
002^Red Blood Cells
|
Leukoreduced
|
2
|
|
Ml
|
|
002^Red Blood Cells
|
Leukoreduced
|
1
|
60
|
Ml
|
|
002^Red Blood Cells
|
Irradiated
|
2
|
15
|
Ml
|
|
002^Red Blood Cells
|
Leukoreduced
|
1
|
|
|
|
020^Platelets
|
Leukoreduced
Irradiated
|
6
|
|
|
|
024^ Apheresis Platelets
|
Irradiated
|
1
|
|
|
|
002^Red Blood Cells
|
|
1
|
|
|
|
Factor VIII
|
|
2
|
910
|
IU
|
HL7 Attribute Table - BPO - Blood product order
4.14.1.0 BPO field definitions
4.14.1.1 BPO-1 Set ID - BPO (SI) 01700
Definition: This field contains the sequence number for the BPO segment within the message. For the first order transmitted, the sequence number shall be 1; for the second order, it shall be 2; and so on.
4.14.1.2 BPO-2 BP Universal Service Identifier (CWE) 01701
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identifier code for the requested blood product. This can be based on local and/or "universal" codes. We recommend the "universal" procedure identifier. The structure of this CWE data type is described in the control section. The preferred coding system is the ISBT 128 Product Code.
Blood Product Orders for commercial products, such as Rh Immune Globulin or Factor VIII concentrate, are not at this time defined in an international or national coding system as are blood products. Therefore, locally defined codes can be used for the Universal Service Identifier for commercial products.
4.14.1.3 BPO-3 BP Processing Requirements (CWE) 01702
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains additional information about the blood component class associated with the Universal Service ID. The placer of the order can specify any required processing of the blood product that must be completed prior to transfusion to the intended recipient. Refer to User-Defined Table 0508 - Blood Product Processing Requirements in Chapter 2C, Code Tables, for suggested values.
4.14.1.4 BPO-4 BP Quantity (NM) 01703
Definition: This field contains the number of blood products ordered.
4.14.1.5 BPO-5 BP Amount (NM) 01704
Definition: This field contains the ordered amount (volume) associated with each quantity of blood product.
4.14.1.6 BPO-6 BP Units (CWE) 01705
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the units of measure for the blood product amount. (See Chapter 7 for more details about reporting units.) This field specifies the units of measure for volume of a blood component (i.e., 50 ml) or the units of measure or dosage of a commercial product (i.e., 910 I.U. - International Units - of Factor VIII Concentrate).
4.14.1.7 BPO-7 BP Intended Use Date/Time (DTM) 01706
Definition: This field specifies the date/time that the placer intends to use the blood product that is being ordered.
This is the time when the placer expects the product to be available within the transfusion service. For example, the product should be available for use, but not dispensed, on this date/time.
4.14.1.8 BPO-8 BP Intended Dispense From Location (PL) 01707
Components: <Point of Care (HD)> ^ <Room (HD)> ^ <Bed (HD)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Person Location Type (IS)> ^ <Building (HD)> ^ <Floor (HD)> ^ <Location Description (ST)> ^ <Comprehensive Location Identifier (EI)> ^ <Assigning Authority for Location (HD)>
Subcomponents for Point of Care (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Room (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Bed (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Building (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Floor (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Comprehensive Location Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Authority for Location (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the location from which the blood component is to be dispensed.
4.14.1.9 BPO-9 BP Intended Dispense From Address (XAD) 01708
Components: <Street Address (SAD)> ^ <Other Designation (ST)> ^ <City (ST)> ^ <State or Province (ST)> ^ <Zip or Postal Code (ST)> ^ <Country (ID)> ^ <Address Type (ID)> ^ <Other Geographic Designation (ST)> ^ <County/Parish Code (CWE)> ^ <Census Tract (CWE)> ^ <Address Representation Code (ID)> ^ <WITHDRAWN Constituent> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Temporary Indicator (ID)> ^ <Bad Address Indicator (ID)> ^ <Address Usage (ID)> ^ <Addressee (ST)> ^ <Comment (ST)> ^ <Preference Order (NM)> ^ <Protection Code (CWE)> ^ <Address Identifier (EI)>
Subcomponents for Street Address (SAD): <Street or Mailing Address (ST)> & <Street Name (ST)> & <Dwelling Number (ST)>
Subcomponents for County/Parish Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Census Tract (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Address Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the actual address of the location from which the blood component is to be dispensed.
4.14.1.10 BPO-10 BP Requested Dispense Date/Time (DTM) 01709
Definition: This field specifies the date/time that the requested blood products must be ready to dispense. This date/time may be different from the intended use date/time. For example, the patient may be scheduled to come in for a transfusion at a specified time. However, the placer would request that the blood product be ready to dispense prior to that time in order to have the blood component ready for transfusion at the scheduled time. The field may also be used to indicate that the placer is now ready to pick up the ordered blood product and is requesting the blood product be ready to dispense at that time.
4.14.1.11 BPO-11 BP Requested Dispense to Location (PL) 01710
Components: <Point of Care (HD)> ^ <Room (HD)> ^ <Bed (HD)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Person Location Type (IS)> ^ <Building (HD)> ^ <Floor (HD)> ^ <Location Description (ST)> ^ <Comprehensive Location Identifier (EI)> ^ <Assigning Authority for Location (HD)>
Subcomponents for Point of Care (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Room (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Bed (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Building (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Floor (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Comprehensive Location Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Authority for Location (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the inpatient or outpatient location to which the blood component is to be dispensed. The default dispense to location is the current census location for the patient.
4.14.1.12 BPO-12 BP Requested Dispense to Address (XAD) 01711
Components: <Street Address (SAD)> ^ <Other Designation (ST)> ^ <City (ST)> ^ <State or Province (ST)> ^ <Zip or Postal Code (ST)> ^ <Country (ID)> ^ <Address Type (ID)> ^ <Other Geographic Designation (ST)> ^ <County/Parish Code (CWE)> ^ <Census Tract (CWE)> ^ <Address Representation Code (ID)> ^ <WITHDRAWN Constituent> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Temporary Indicator (ID)> ^ <Bad Address Indicator (ID)> ^ <Address Usage (ID)> ^ <Addressee (ST)> ^ <Comment (ST)> ^ <Preference Order (NM)> ^ <Protection Code (CWE)> ^ <Address Identifier (EI)>
Subcomponents for Street Address (SAD): <Street or Mailing Address (ST)> & <Street Name (ST)> & <Dwelling Number (ST)>
Subcomponents for County/Parish Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Census Tract (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Address Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the actual address of the location to which the blood component is to be dispensed. The default dispense to location is the current census location for the patient.
4.14.1.13 BPO-13 BP Indication for Use (CWE) 01712
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This is a coded optional field. The value indicates the reason that the blood product was ordered. This information is helpful for prospective review or retrospective studies of blood product ordering practices of the ordering provider by the Quality Assurance Department and/or Transfusion Committee. Refer to User-Defined Table 0509 - Indication for Use in Chapter 2C, Code Tables, for suggested values.
4.14.1.14 BPO-14 BP Informed Consent Indicator (ID) 01713
This field indicates whether consent for the transfusion has been obtained. Refer to HL7 table 0136 -Yes/No indicator as defined in Chapter 2C, Code Tables.
4.14.2 BPX - Blood Product Dispense Status Segment
In the processing of blood products, it is necessary for the transfusion service and the placer system to communicate information. The status messages need to contain additional information regarding the blood products requested, such as the unique donation ID, product code, blood type, expiration date/time of the blood product, and current status of the product. This segment is similar to an OBX segment, but contains additional attributes.
HL7 Attribute Table - BPX - Blood product dispense status
4.14.2.0 BPX field definitions
The BP prefix in the element name indicates that the attribute pertains to any type of blood product. A blood product is defined as any type of blood component or commercially prepared blood product that is prepared and dispensed from the transfusion service.
The BC prefix in the element name indicates that the attribute pertains only to blood components. A blood component is defined as the whole or any part of a blood donation. For example, from one whole blood donation, the unit of whole blood can be fractionated into red blood cells, plasma and platelets with each component contained in separate bags. These types of blood products are assigned a unique donation identification number as well as a product code that indicates the type of component contained in the bag.
The CP prefix in the element name indicates that the attribute pertains only to Commercial Products. A commercial product is defined as a commercially manufactured product, such as blood derivatives ( Rh Immune Globulin, Factor VIII Concentrate or Blood Recipient Sets or Filters). These types of products are tracked by manufacturer and lot number and are not necessarily assigned a unique donation number.
4.14.2.1 BPX-1 Set ID - BPX (SI) 01714
Definition: This field contains the sequence number for the BPX segment under the related BPO segment. For the first blood product dispense status transmitted, the sequence number shall be 1; for the second product dispense status, it shall be 2; and so on.
4.14.2.2 BPX-2 BP Dispense Status (CWE) 01715
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the current status of the specified blood product as indicated by the filler or placer. For example, the first status change of a product that may trigger a Blood Product Dispense Status Message occurs when it first becomes linked to a patient and is ready to dispense. The placer system may use the Blood Product Dispense Status Message to request the transfusion service to dispense the product. When the blood product is delivered or issued to a patient, the status of the blood product would be changed to indicate that it has now been "dispensed." Refer to HL7 Table 0510 - Blood Product Dispense Status in Chapter 2C, Code Tables, for valid entries.
4.14.2.3 BPX-3 BP Status (ID) 01716
Definition: The most commonly used message status values in a BPX will be preliminary and final. A status is considered preliminary until a blood product has reached a final disposition for the patient. For example, when the product is first cross-matched and a status message is sent, it would be considered preliminary. When the product is dispensed to the patient, that status would also be considered preliminary. However, once the product is transfused, the status would be considered final. The status of a blood product (BPX-2) can continue to change and the previous status should be overwritten until it reaches a final status (BPX-3). Refer to HL7 Table 0511 - BP Observation Status Codes Interpretation in Chapter 2C, Code Tables, for valid entries.
4.14.2.4 BPX-4 BP Date/Time of Status (DTM) 01717
Definition: This field indicates the date and time that the status of the blood component was changed. For example, if the blood component had a status, of "RD" (Ready to Dispense), the date and time in this field would indicate the date and time that component was made ready to dispense by the filler system.
4.14.2.5 BPX-5 BC Donation ID (EI) 01718
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The Donation ID is the unique identification number assigned to a blood donation. The Donation ID depends upon the bar code labeling system used for the component. There are currently two blood component labeling standards: ABC CODABAR and ISBT 128. The preferred labeling system is ISBT 128. If using ISBT 128, the Donation ID is an internationally unique identifier consisting of the following 13 characters:
Country Code & Collection Facility - 5 characters
Donation Year - 2 characters
Serial Number - 6 characters
This field is required for blood components and is not applicable for commercial product messages.
4.14.2.6 BPX-6 BC Component (CNE) 01719
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The Component field includes an identifier and description of the specific blood component.
The identifier consists of a numeric or alphanumeric product code that represents the type of blood component. The coding system will be determined by the bar code labeling system on the particular component of blood. The preferred coding system is ISBT 128.
If using ISBT 128 labeling standard, the product code will consist of an 8-character alphanumeric code, starting with an alpha character and including the component class, donation type/intended use and division indicator.
If using CODABAR product labeling standard, the product code is a 5-digit number.
This field is required for blood components and is not applicable for commercial product messages.
4.14.2.7 BPX-7 BC Donation Type / Intended Use (CNE) 01720
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the type of donation or collection/intended use. This value is populated from Table 5 -Type of Donation in the ISBT 128 Application Specification. The default value is "0", meaning "Not specified." Other values indicate whether the blood product (1) is an allogeneic unit from a volunteer donor, (2) is intended for a specific recipient but may be crossed over and used for another recipient, or (3) is an autologous donation intended only for that particular recipient.
This field is optional for blood component messages and is not applicable for commercial product messages.
4.14.2.8 BPX-8 CP Commercial Product (CWE) 01721
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the code and/or text to identify a commercial product. Examples of commercial products are blood derivatives such as Rh Immune Globulin and Factor VIII concentrate, Leukoreduction filters, and blood administration sets.
Either code and/or text may be absent. However, the code is always placed in the first component position and any free text in the second component. Thus, a component delimiter must precede free text without a code. Free text can be utilized if no update is to occur. Refer To User-Defined Table 0512 - Commercial Product in Chapter 2C, Code Tables, for suggested values.
This field is required for commercial blood products and is not applicable for blood component messages.
4.14.2.9 BPX-9 CP Manufacturer (XON) 01722
Components: <Organization Name (ST)> ^ <Organization Name Type Code (CWE)> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Organization Identifier (ST)>
Subcomponents for Organization Name Type Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field identifies the manufacturer of the commercial product. The manufacturer may be different from the supplier of the commercial product.
This field is required for commercial blood products and is not applicable for blood component messages.
4.14.2.10 BPX-10 CP Lot Number (EI) 01723
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field identifies the lot number for blood derivatives or commercially supplied items used as accessories to transfusion.
This field is required for commercial blood products and is not applicable for blood component messages.
4.14.2.11 BPX-11 BP Blood Group (CNE) 01724
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the ABO/Rh blood group of the blood component. The preferred values for the blood group are the specified values in Table 3A - Encodation of ABO/Rh Blood Group in the ISBT 128 Application Specification.
This field is required for blood components and certain commercial products (such as solvent detergent plasma).
4.14.2.12 BPX-12 BC Special Testing (CNE) 01725
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This is a repeating field to allow multiple entries for special testing that was performed on the blood component. The preferred coding system for Special Testing is defined in the ISBT 128 Application Specification. Proposals have been developed and will soon be published by ICCBBA, Inc. for the encodation of other antigen and antibody specificities, including HLA, platelet, red cell and other types of markers.
This field is optional for blood component messages. It is not applicable for non-commercial product messages.
Refer to Table I3 - Special Testing Codes of the ISBT 128 Application Specification.
4.14.2.13 BPX-13 BP Expiration Date/Time (DTM) 01726
Definition: This field specifies the date and time that the blood product expires. The blood product is no longer considered acceptable once the expiration date has been reached unless cleared by the transfusion service medical staff.
This field applies to blood components as well as commercial products. There are a few commercial products that have no expiration date. Therefore, the field is not required for those specific products.
4.14.2.14 BPX-14 BP Quantity (NM) 01727
Definition: This field indicates the number of blood components or commercial products to which this message refers.
4.14.2.15 BPX-15 BP Amount (NM) 01728
Definition: This field contains the ordered amount (volume) associated with each quantity of a blood component or commercial product to which this message refers.
4.14.2.16 BPX-16 BP Units (CWE) 01729
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the units of measure for the blood product amount. (See Chapter 7 for more details about reporting units.) This field specifies the units of measure for volume of a blood component (i.e., 50 ml) or the units of measure or dosage of a commercial product (i.e., 910 I.U. - International Units - of Factor VIII Concentrate).
4.14.2.17 BPX-17 BP Unique ID (EI) 01730
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is a unique system-generated number assigned to the blood product to which the message is referring. Each time the status is updated, the new message should replace the previous message if the Blood Product Unique ID is the same. If the Blood Product Unique ID is different, it indicates that the status applies to a different blood product.
The sending and receiving systems must agree upon the use of this field.
4.14.2.18 BPX-18 BP Actual Dispensed to Location (PL) 01731
Components: <Point of Care (HD)> ^ <Room (HD)> ^ <Bed (HD)> ^ <Facility (HD)> ^ <Location Status (IS)> ^ <Person Location Type (IS)> ^ <Building (HD)> ^ <Floor (HD)> ^ <Location Description (ST)> ^ <Comprehensive Location Identifier (EI)> ^ <Assigning Authority for Location (HD)>
Subcomponents for Point of Care (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Room (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Bed (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Building (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Floor (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Comprehensive Location Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Authority for Location (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the inpatient or outpatient location to which the blood product was actually dispensed. The default value is the current census location for the patient.
4.14.2.19 BPX-19 BP Actual Dispensed to Address (XAD) 01732
Components: <Street Address (SAD)> ^ <Other Designation (ST)> ^ <City (ST)> ^ <State or Province (ST)> ^ <Zip or Postal Code (ST)> ^ <Country (ID)> ^ <Address Type (ID)> ^ <Other Geographic Designation (ST)> ^ <County/Parish Code (CWE)> ^ <Census Tract (CWE)> ^ <Address Representation Code (ID)> ^ <WITHDRAWN Constituent> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Expiration Reason (CWE)> ^ <Temporary Indicator (ID)> ^ <Bad Address Indicator (ID)> ^ <Address Usage (ID)> ^ <Addressee (ST)> ^ <Comment (ST)> ^ <Preference Order (NM)> ^ <Protection Code (CWE)> ^ <Address Identifier (EI)>
Subcomponents for Street Address (SAD): <Street or Mailing Address (ST)> & <Street Name (ST)> & <Dwelling Number (ST)>
Subcomponents for County/Parish Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Census Tract (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Expiration Reason (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Protection Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Address Identifier (EI): <Entity Identifier (ST)> & <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field contains the actual address of the location to which the blood product was actually dispensed.
4.14.2.20 BPX-20 BP Dispensed to Receiver (XCN) 01733
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This is the person who picked up and transported the blood component(s) or commercial product(s). The code for the receiver is recorded as a XCN data type. This field can be free text. In this case, the receiver's name must be recorded as the second through fourth components of the field.
4.14.2.21 BPX-21 BP Dispensing Individual (XCN) 01734
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field identifies the individual who is dispensing the blood component or commercial product.
4.14.3 BTX - Blood Product Transfusion/Disposition Segment
HL7 Attribute Table - BTX - Blood Product Transfusion/Disposition
4.14.3.0 BTX field definitions
The BP prefix in the element name indicates that the attribute pertains to any type of blood product. A blood product is defined as any type of blood component or commercially prepared blood product that is prepared and dispensed from the transfusion service.
The BC prefix in the element name indicates that the attribute pertains only to blood components. A blood component is defined as any part or all of a whole blood donation. For example, from one whole blood donation, the unit of whole blood can be fractionated into red blood cells, plasma and platelets with each component contained in separate bags. These types of blood products are always assigned a unique donation identification number as well as a product code that indicates the type of component contained in the bag.
The CP prefix in the element name indicates that the attribute pertains only to Commercial Products. A commercial product is defined as a commercially manufactured product, such as blood derivatives (Rh Immune Globulin, Factor VIII Concentrate or Blood Recipient Sets or Filters). These types of products are tracked by manufacturer and lot number and are not necessarily assigned a unique donation number.
4.14.3.1 BTX-1 Set ID - BTX (SI) 01735
Definition: This field contains the sequence number for the BTX segment under the related BPO segment. For the first product transfusion/disposition transmitted, the sequence number shall be 1; for the second product transfusion/disposition, it shall be 2; and so on.
4.14.3.2 BTX-2 BC Donation ID (EI) 01736
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The donation ID is the unique identification number assigned to a blood donation. The Donation ID depends upon the bar code labeling system used for the component. There are currently two blood component labeling standards: ABC CODABAR and ISBT 128. The preferred labeling system is ISBT 128. If using ISBT 128, the Donation ID is an internationally unique identifier consisting of the following 13 characters:
Country Code & Collection Facility - 5 characters
Donation Year - 2 characters
Serial Number - 6 characters
This is required for blood components and is not applicable for commercial product messages.
4.14.3.3 BTX-3 BC Component (CNE) 01737
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The Blood Component field includes an identifier and description of the specific blood component.
The identifier consists of a numeric or alphanumeric product code that represents the type of blood component. The coding system will be determined by the bar code labeling system on the particular component of blood. The preferred coding system is ISBT 128.
If using ISBT 128 labeling standard, the product code will consist of an 8-character alphanumeric code, starting with an alpha character and including the component class, donation type/intended use and division indicator.
If using CODABAR product labeling standard, the product code is a 5-digit number.
This field is required for blood components and is not applicable for commercial product messages.
4.14.3.4 BTX-4 BC Blood Group (CNE) 01738
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the ABO/Rh blood group of the blood component. The preferred values for the blood group are the specified values in Table 3A - Encodation of ABO/Rh Blood Group in the ISBT 128 Application Specification.
This field is required for blood components and certain commercial products (such as solvent detergent plasma).
4.14.3.5 BTX-5 CP Commercial Product (CWE) 01739
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the code and/or text to identify a commercial product. Examples of commercial products are blood derivatives such as Rh Immune Globulin and Factor VIII concentrate, Leukoreduction filters, and blood administration sets.
Either code and/or text may be absent. However, the code is always placed in the first component position and any free text in the second component. Thus, free text without a code must be preceded by a component delimiter. Free text can be utilized if no update is to occur. Refer to User-Defined Table 0512 - Commercial Product in Chapter 2C, Code Tables, for suggested values.
This field is required for commercial blood products and is not applicable to blood component messages.
4.14.3.6 BTX-6 CP Manufacturer (XON) 01740
Components: <Organization Name (ST)> ^ <Organization Name Type Code (CWE)> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Organization Identifier (ST)>
Subcomponents for Organization Name Type Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: This field identifies the manufacturer of the commercial product. The manufacturer may not be the same as the supplier of the commercial product.
This field is required for commercial blood products and is not applicable for blood component messages.
4.14.3.7 BTX-7 CP Lot Number (EI) 01741
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field identifies the lot number for blood derivatives or commercially supplied items used as accessories to transfusion.
This field is required for commercial blood products and is not applicable for blood component messages.
4.14.3.8 BTX-8 BP Quantity (NM) 01742
Definition: This field indicates the number of blood components or commercial products to which the message refers.
4.14.3.9 BTX-9 BP Amount (NM) 01743
Definition: This field contains the amount (volume) associated with each blood component or commercial product. When included in this segment, it may be used to indicate the volume of the blood component or product that was actually transfused.
4.14.3.10 BTX-10 BP Units (CWE) 01744
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the units of measure for the blood component or commercial product amount. (See Chapter 7 for more details about reporting units.) This specifies the units of measure for volume of a blood component (i.e., 50 ml) or the units of measure or dosage of a commercial product (i.e., 910 I.U. - International Units - of Factor VIII Concentrate).
4.14.3.11 BTX-11 BP Transfusion/Disposition Status (CWE) 01745
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field indicates the current status of the specified blood product as indicated by the placer. For example, the placer may return the blood product to the transfusion service unused because an IV could not be started. The blood component may have been entered, but the line was clogged and could not be used, in which case the component must be wasted. A final status would indicate that the product has actually been "transfused." Refer to HL7 Table 0513 - Blood Product Transfusion/Disposition Status in Chapter 2C, Code Tables, for suggested values.
4.14.3.12 BTX-12 BP Message Status (ID) 01746
Definition: The most commonly used message status values in a BTX will be preliminary and final. A status is considered preliminary until a blood product has reached a final disposition for the patient. For example, when the product is first cross-matched and a status message is sent, it would be considered preliminary. When the product is dispensed to the patient, that status would also be considered preliminary. However, once the product is transfused, the status would be considered final. The status of a blood product (BTX-11) can continue to change and the previous result should be overwritten until it reaches a final status (BTX-12). Refer to HL7 Table 0511 - BP Observation Status Codes Interpretation in Chapter 2C, Code Tables, for valid entries.
4.14.3.13 BTX-13 BP Date/Time of Status (DTM) 01747
Definition: This field indicates the date and time that the status of the blood component was changed. For example, if the blood component had a status of "TX" (Transfused), the date and time in this field would indicate the date and time the component was transfused by the placer system.
4.14.3.14 BTX-14 BP Transfusion Administrator (XCN) 01748
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identity of the individual who administers the transfusion of the blood product. If the code is sent as a local code, it should be unique and unambiguous. This field can be free text to permit capture without table update. In this case, the administrator's name must be recorded as the second through fourth components of the field.
4.14.3.15 BTX-15 BP Transfusion Verifier (XCN) 01749
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the identity of the individual who assists in the identification of the patient and verification of the product information prior to transfusion of the blood product. If the ID Number is sent as a local code, it should be unique and unambiguous. This field can be free text to permit capture without table update. In this case, the verifier's name must be recorded as the second through fourth components of the field.
4.14.3.16 BTX-16 BP Transfusion Start Date/Time of Status (DTM) 01750
Definition: This field indicates the date and time that the administrator started the transfusion of the blood component or commercial product.
4.14.3.17 BTX-17 BP Transfusion End Date/Time of Status (DTM) 01751
Definition: This field indicates the date and time that the transfusion of the blood component or commercial product was completed or stopped.
4.14.3.18 BTX-18 BP Adverse Reaction Type (CWE) 01752
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the type of adverse reaction that the recipient of the blood product experienced. Refer to User-Defined Table 0514 - Transfusion Adverse Reaction in Chapter 2C, Code Tables, for suggested values.
4.14.3.19 BTX-19 BP Transfusion Interrupted Reason (CWE) 01753
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: This field contains the reason that the transfusion of the blood product was interrupted. Refer to User-Defined Table 0515 - Transfusion Interrupted Reason in Chapter 2C, Code Tables, for suggested values.
4.14.3.20 BTX-20 BP Unique ID (EI) 03391
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: This field is a unique system-generated number assigned to the blood product to which the message is referring. Each time the status is updated, the new message should replace the previous message if the Blood Product Unique ID is the same. If the Blood Product Unique ID is different, it indicates that the status applies to a different blood product.
4.15 TRANSFUSION SERVICE (BLOOD BANK) TRANSACTION FLOW DIAGRAM
The following diagram depicts the message flow of the blood product messages.







4.16 DONATION SERVICE (BLOOD BANK) TRIGGER EVENTS AND MESSAGES
4.16.1 Usage Notes for Donation Service (Blood Bank)
The Donation Service (BLOOD BANK) uses a different methodology than the similar Transfusion Service (BLOOD BANK) already present in this standard. Each of the segments defined for the Transfusion Service groups together all the 'transfusion' information in one segment, each. The Donation Service was developed breaking out the blood product 'donated' from the donation event itself. This is a more sustainable and interoperable approach. Future changes to the Transfusion Service should uptake this style.
4.16.2 Activity Diagram
The donation service messages facilitate communications between typical system components in a blood bank donation service facility. Frequently different components of blood banking systems (e.g. registration, questionnaire) are bundled together in one system produced by one vendor. However since there is no standard for that bundling, in any particular implementation any of the named system components can be implemented on another system and therefore communications to that component is necessary. The typical components are illustrated in the graphic below.
Additionally, the graphic also depicts a flow of information through those systems during a donation process.
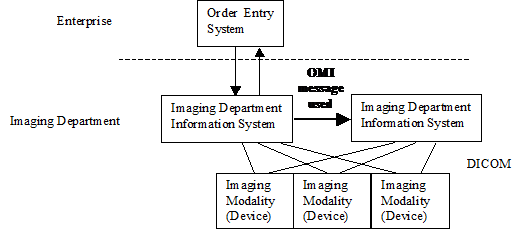
As mentioned previously, many of the existing systems used in the collection process conduct all these actions in a single bundled system. Extension of the systems on this page is presented in this format because there is no standard for that bundling, in any particular implementation any of the named system components can be implemented on another system and therefore communications to that component is necessary.
4.16.3.0 Ordering Provider
For Directed and Autologous Donations, this is the Healthcare Provider requesting a blood donation.
4.16.3.1 Registration System
All donors are registered in this system.
4.16.3.2 Donor book of record System
This is the source-of-truth for every donor, whether evaluated and deferred, rejected, or not deferred.
4.16.3.3 Mini-physical System
The mini-physical examination conducted on all potential donors is documented using this system.
4.16.3.4 Questionnaire System
Each potential donor must fill out a questionnaire which asks about previous medical history and risk factors using this documentation system.
4.16.3.5 Donation System
The phlebotomists and other healthcare professionals use this system to document the blood donation procedure.
4.16.3.6 Device Interfaces
Interface to devices used during the mini-physical, donation, and shipping systems.
4.16.3.7 Provider Master
This system keeps the master list of providers.
4.16.3.8 Shipping System
This system is used to document the shipping manifest from information received from the actual donations.
4.16.4 DBC - Create Donor Record Message (Event O41 )
The Create Donor Record messages contain information to create a new donor book of record.
DBC^O41^DBC_O41: Create Donor Record Message
Status
Chapter
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
|
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
]
|
--- DONOR end
|
|
4.16.5 DBU - Update Donor Record Message (Event O42)
The Update Donor Record messages contain information to update an existing donor book of record.
DBU^O42^DBC_O42: Update Donor Record Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
]
|
--- DONOR end
|
|
|
|
4.16.6 QBP - Get Donor Record Candidates (Event Q33)
This query/response is designed for interaction between a registration system and the system which contains the Donor Book of Record. The query consists of query parameters which assist in determining if the Donor already has a record in the Donor Book or Record system. The query parameters are minimal and number of elements returned in the query response for each candidate is minimal.
4.16.7 RSP - Get Donor Record Candidates Response (K33)
RSP^K33^RSP_O33: Get Donor Record Candidates Response Message
|
Segment
|
Descriptions
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
MSA
|
Message Acknowledgement
|
|
|
DB |
|
[
ERR
]
|
Error
|
|
|
DB |
|
QAK
|
Query Acknowledgement
|
|
|
DB |
|
QPD
|
Query Parameter Definition
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
|
3
|
DB |
|
]
|
--- DONOR end
|
|
|
|
|
|
4.16.8 QBP - Get Donor Record (Event Q34)
This query/response is designed for interaction between a viewing system and the system which contains the Donor Book of Record. The query consists of query parameters, and the response of the demographics for that donor.
4.16.9 RSP - Get Donor Record Response (K34)
RSP^K34^RSP_O34: Segment Pattern Response Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
MSA
|
Message Acknowledgement
|
|
|
DB |
|
[
ERR
]
|
Error
|
|
|
DB |
|
QAK
|
Query Acknowledgement
|
|
|
DB |
|
QPD
|
Query Parameter Definition
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
--- DONOR end
|
|
|
|
|
[
|
--- DONATION begin
|
|
|
|
|
DON
|
Donation
|
|
|
DB |
|
[
{
OBX
}
]
|
Adverse Reaction Observations
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (For Donation)
|
DB |
|
]
|
--- DONATION end
|
|
|
|
4.16.10 DRG - Donor Registration (Event O43)
The Donor Registration messages contain information to register a donor for a donation.
DRG^O43^DRG_O43: Donor Registration Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
--- DONOR end
|
|
|
|
4.16.11 DER - Donor Eligibility Request (Event O44)
The Donor Registration messages contain minimal information about a donor registration.
DER^O44^DER_O44: Donor Registration - Minimal Message
|
Segment
|
Description
|
Satus
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
--- DONOR end
|
|
|
|
|
{
|
--- DONOR_ORDER begin
|
|
|
|
|
OBR
|
Observation
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Detail)
|
DB |
|
}
|
--- DONOR_ORDER end
|
|
|
|
4.16.12 DEO - Donor Eligibility Observations (Event O45)
Communicate both mini-physical observations and questions and answers from a donor questionnaire.
DEO^O45^DEO_O45: Donor Eligibility Observations Message
|
Segment
|
Description
|
Satus
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- Donor begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
|
|
{
|
--- DONATION_ORDER begin
|
|
|
OBR
|
Observations Request
|
DB |
|
[
{
NTE
}
]
|
Notes and comments
|
DB |
|
[{
|
--- DONATION_OBSERVATION begin
|
|
|
OBX
|
Observation related to OBR
|
DB |
|
[
{
NTE
}
]
|
Notes and comments
|
DB |
|
}]
|
--- DONATION_OBSERVATION end
|
|
|
}
|
--- DONATION_ORDER end
|
|
|
|
4.16.13 DEL - Donor Eligibility (Event O46)
Use this message to communicate a donor's eligibility to donate.
DEL^O46^DEL_O46: Donor Eligibility Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
|
|
DON
|
|
|
[
{
NTE
}
]
|
Notes and Comments (for Donation)
|
DB |
4.16.14 DRC - Donor Request to Collect (Event O47)
Used to communicate to a collection system that the donor is eligible and collection can begin.
DRC^O47^DRC_O47: Donor Request to Collect Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
|
|
{
|
|
|
OBR
|
|
|
[
{
NTE
}
]
|
|
|
}
|
|
4.16.15 DPR - Donation Procedure (Event O48)
This message contains information from the blood unit collection procedure from the donor.
DPR^O48^DPR_O48: Donation Procedure Message
|
Segment
|
Description
|
Status
|
Chapter
|
DB |
|
MSH
|
Message Header
|
|
|
DB |
|
[
{
SFT
}
]
|
Software Segment
|
|
|
DB |
|
[
UAC
]
|
User Authentication Credential
|
|
|
DB |
|
[
|
--- DONOR begin
|
|
|
|
|
PID
|
Patient Identification Segment
|
|
|
DB |
|
[
PD1
]
|
Additional Demographics
|
DB |
|
[
{
OBX
}
]
|
Donor Observations
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor)
|
DB |
|
[
{
AL1
}
]
|
Allergy Information
|
DB |
|
[
{
ARV
}
]
|
Access Restrictions
|
DB |
|
[
|
--- DONOR_REGISTRATION begin
|
|
|
|
|
[
PV1
]
|
Patient Visit (Donor Registration)
|
|
|
DB |
|
[
{
NTE
}
]
|
Notes and Comments (for Donor Registration)
|
DB |
|
]
|
--- DONOR_REGISTRATION end
|
|
|
|
|
]
|
|
|
{
|
|
|
OBR
|
|
|
[
{
NTE
}
]
|
|
|
}
|
|
|
[
|
--- DONATION begin
|
|
|
|
|
DON
|
|
|
[
{
OBX
}
]
|
|
|
[
{
NTE
}
]
|
Notes and Comments (for Donation)
|
DB |
|
[
|
--- BLOOD_UNIT begin
|
|
|
|
|
[
{
BUI
}
]
|
|
|
[
{
NTE
}
]
|
Notes and Comments (for Blood Unit)
|
DB |
|
]
|
--- BLOOD_UNIT end
|
|
|
|
|
]
|
--- DONATION end
|
|
|
|
4.17 DONATION SERVICE (BLOOD BANK) SEGMENTS
4.17.1 DON - Donation Segment
The intent of this segment is to describe the actual donation procedure.
HL7 Attribute Table - DON - Donation
4.17.1.0 DON field Definitions
4.17.1.1 DON-1 Donation Identification Number - DIN (EI) 03340
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
4.17.1.2 Definition: This field contains a unique identifier, Donation Identification Number (DIN), for the specific donation and is therefore mandatory except when using an eligibility message type in which only DON-9, DON-10, and DON-11 are populated. DON-2 Donation Type (CNE) 03341
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The type of donation. This element is mandatory except when using an eligibility message type in which only DON-9, DON-10, and DON-11 are populated. The values for this field are defined in Table RT008 - Type of Donation or Collection in 6th Position of Product Code in the ISBT 128 Standard Technical Specification, which is maintained by ICCBBA. Link: http://iccbba.org/technicalspecification.pdf. Table 5 Data Structure 002.
4.17.1.3 DON-3 Phlebotomy Start Date/Time (DTM) 03342
Definition: The start date and time of the phlebotomy.
4.17.1.4 DON-4 Phlebotomy End Date/Time (DTM) 03343
Definition: The end date and time of the phlebotomy.
4.17.1.5 DON-5 Donation Duration (NM) 03344
Definition: The duration of the phlebotomy or the length of time that elapsed between the phlebotomy start date and time and the phlebotomy end date and time.
4.17.1.6 DON-6 Donation Duration Units (CNE) 03345
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The duration units. The duration units and duration are restricted to minutes and seconds. Concepts are pulled from the UCUM code system (www.unitsofmeasure.org). Refer to HL7-Defined Table 0932 - Donation Duration Units in Chapter 2C, Code Tables, for valid entries.
4.17.1.7 DON-7 Intended Procedure Type (CNE) 03346
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The procedure(s) intended to be performed on the donor. Refer to HL7-Defined Table 0933 - Intended Procedure Type in Chapter 2C, Code Tables, for valid entries.
4.17.1.8 DON-8 Actual Procedure Type (CNE) 03347
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The actual procedure(s) performed on the donor. Refer to HL7-Defined Table 0933 - Intended Procedure Type in Chapter 2C, Code Tables, for valid entries.
4.17.1.9 DON-9 Donor Eligibility Flag (ID) 03348
Definition: Is the Donor eligible for donation? Yes or No. Refer to HL7 Table 0136 -Yes/No Indicator as defined in Chapter 2C, Code Tables.
4.17.1.10 DON-10 Donor Eligibility Procedure Type (CNE) 03349
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The procedure(s) for which the donor is eligible. Refer to HL7-Defined Table 0933 - Intended Procedure Type in Chapter 2C, Code Tables, for valid entries.
4.17.1.11 DON-11 Donor Eligibility Date (DTM) 03350
Definition: The date and time on which the donor is eligible to donate.
4.17.1.12 DON-12 Process Interruption (CNE) 03351
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Was the donation process interrupted after it began? Refer to HL7-Defined Table 0923 - Process Interruption in Chapter 2C, Code Tables, for valid entries.
4.17.1.13 DON-13 - Process Interruption Reason (CNE) 03352
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Reason that the donation process was interrupted. Refer to HL7-Defined Table 0935 - Process Interruption Reason in Chapter 2C, Code Tables, for valid entries.
4.17.1.14 DON-14 Phlebotomy Issue (CNE) 03353
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Indicates whether there is a problem or issue with the phlebotomy itself. This may be due to an incorrect needle procedure, needle defect, tube blockage, problem with the apheresis machine, or improper action by the phlebotomist. Refer to HL7-Defined Table 0925 - Phlebotomy Issue in Chapter 2C, Code Tables, for valid entries.
4.17.1.15 DON-15 Intended Recipient Blood Relative (ID) 03354
Definition: If this donation has an intended recipient (directed, dedicated, designated), is the intended recipient a blood relative of the donor? Yes or No. Refer to HL7 Table 0136 -Yes/No Indicator as defined in Chapter 2C, Code Tables.
4.17.1.16 DON-16 Intended Recipient Name (XPN) 03355
Components: <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <Name Type Code (ID)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Called By (ST)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: If this is donation has an intended recipient (autologous, directed, dedicated, designated), the intended recipient's name.
4.17.1.17 DON-17 Intended Recipient DOB (DTM) 03356
Definition: If this donation has an intended recipient (autologous, directed, dedicated, designated), the intended recipient's date of birth.
4.17.1.18 DON-18 Intended Recipient Facility (XON) 03357
Components: <Organization Name (ST)> ^ <Organization Name Type Code (CWE)> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Organization Identifier (ST)>
Subcomponents for Organization Name Type Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: If this donation has an intended recipient (autologous, directed, dedicated, designated), the facility where the intended recipient is expected to receive the transfusion.
4.17.1.19 DON-19 Intended Recipient Procedure Date (DTM) 03358
Definition: If this donation has an intended recipient (autologous, directed, dedicated, designated), the date the intended recipient is expected to receive the transfusion.
4.17.1.20 DON-20 Intended Recipient Ordering Provider (XPN) 03359
Components: <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <Name Type Code (ID)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Called By (ST)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: If this is donation has an intended recipient (autologous, directed, dedicated, designated), the provider who ordered the directed donation for the intended recipient.
4.17.1.21 DON-21 Phlebotomy Status (CNE) 03360
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: Whether the phlebotomy was successful, not drawn, or unsuccessful, and if unsuccessful, the extent to which it was unsuccessful. Refer to HL7-Defined Table 0926 - Phlebotomy Status in Chapter 2C, Code Tables, for valid entries.
4.17.1.22 DON-22 Arm Stick (CWE) 03361
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The arm(s) stuck for the donation. Refer to HL7-Defined Table 0927 - Arm Stick in Chapter 2C, Code Tables, for valid entries.
4.17.1.23 DON-23 Bleed Start Phlebotomist (XPN) 03362
Components: <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <Name Type Code (ID)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Called By (ST)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The Phlebotomist who starts the blood flow into the container.
4.17.1.24 DON-24 Bleed End Phlebotomist (XPN) 03363
Components: <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <Name Type Code (ID)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Called By (ST)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The Phlebotomist who ends the blood flow into the container.
4.17.1.25 DON-25 Aphaeresis Type Machine (ST) 03364
Definition: The type of aphaeresis machine, if used, for the donation. It will be the specific product name of the machine (e.g. Trima, Amicus, Alyx, Symal, etc.).
4.17.1.26 DON-26 Aphaeresis Machine Serial Number (ST) 03365
Definition: The serial number of the aphaeresis machine, if used, for the donation.
4.17.1.27 DON-27 Donor Reaction (ID) 03366
Definition: Did the donor have any adverse reaction during the donation procedure? Yes or No. Refer to HL7 Table 0136 -Yes/No Indicator as defined in Chapter 2C, Code Tables. If this element is valued "Y"es, there should be OBX segments following the Donation segment which details the adverse reactions.
4.17.1.28 DON-28 Final Review Staff ID (XPN) 03367
Components: <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <Name Type Code (ID)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Called By (ST)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The Staff member conducting the final review and reconciliation of all documentation created during the collection process.
4.17.1.29 DON-29 Final Review Date/Time (DTM) 03368
Definition: The date and time a final review of all documentation and labeling of the blood material is completed.
4.17.1.30 DON-30 Number of Tubes Collected (NM) 03369
Definition: The number of samples collected during the donation which will be used for subsequent testing.
4.17.1.31 DON-31 Donation Sample Identifier (EI) 03370
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The sample identifier for the sample collected during a donation for the purpose of testing. This is a field for sample or specimen identifiers.
4.17.1.32 DON-32 Donation Accept Staff (XCN) 03371
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The staff member who reviewed all the intake materials, assessments and determined the donor can undergo a donation procedure at this time.
4.17.1.33 DON-32 Donation Material Review Staff (XCN) 03372
Components: <Person Identifier (ST)> ^ <Family Name (FN)> ^ <Given Name (ST)> ^ <Second and Further Given Names or Initials Thereof (ST)> ^ <Suffix (e.g., JR or III) (ST)> ^ <Prefix (e.g., DR) (ST)> ^ <WITHDRAWN Constituent> ^ <DEPRECATED-Source Table (CWE)> ^ <Assigning Authority (HD)> ^ <Name Type Code (ID)> ^ <Identifier Check Digit (ST)> ^ <Check Digit Scheme (ID)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Name Context (CWE)> ^ <WITHDRAWN Constituent> ^ <Name Assembly Order (ID)> ^ <Effective Date (DTM)> ^ <Expiration Date (DTM)> ^ <Professional Suffix (ST)> ^ <Assigning Jurisdiction (CWE)> ^ <Assigning Agency or Department (CWE)> ^ <Security Check (ST)> ^ <Security Check Scheme (ID)>
Subcomponents for Family Name (FN): <Surname (ST)> & <Own Surname Prefix (ST)> & <Own Surname (ST)> & <Surname Prefix from Partner/Spouse (ST)> & <Surname from Partner/Spouse (ST)>
Subcomponents for Source Table (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Name Context (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Jurisdiction (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Agency or Department (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Definition: The staff member who performs review on all documentation subsequent to donation procedure.
4.17.2 BUI - Blood Unit information Segment
The intent of this segment is to describe the information associated with a blood unit, one example of which is one or more blood unit(s) resulting from a donation.
HL7 Attribute Table - BUI - Blood Unit Information
4.17.2.0 BUI field definitions
4.17.2.1 BUI-1 Set ID (SI) 03373
Definition: This field contains a sequence number. When multiple BUI segments are included in the same segment group, this number differentiates between them.
4.17.2.2 BUI-2 Blood Unit Identifier (EI) 03374
Components: <Entity Identifier (ST)> ^ <Namespace ID (IS)> ^ <Universal ID (ST)> ^ <Universal ID Type (ID)>
Definition: The blood unit identifier is a unique identifier assigned to the particular blood unit in a container.
4.17.2.3 BUI-3 Blood Unit Type (CWE) 03375
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The type of blood unit. For donations, this is the type blood unit being described. This element defines which of the types from the referenced table are being described. Refer to HL7-Defined Table 0566 - Blood Unit Type in Chapter 2C, Code Tables, for valid entries.
4.17.2.4 BUI-4 Blood Unit Weight (NM) 03376
Definition: The weight of the blood unit collected, not including the weight of the container.
4.17.2.5 BUI-5 Weight Units (CNE) 03377
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The unit of measure for the weight of the blood unit. Concepts are pulled from the UCUM code system (www.unitsofmeasure.org). Refer to HL7-Defined Table 0929 - Weight Units in Chapter 2C, Code Tables, for valid entries.
4.17.2.6 BUI-6 Blood Unit Volume (NM) 03378
Definition: The volume of the blood unit collected.
4.17.2.7 BUI-7 Volume Units (CNE) 03379
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The unit of measure for the volume of the blood unit. Concepts are pulled from the UCUM code system (Fehler! Hyperlink-Referenz ungültig.Refer to HL7-Defined Table 0930 - Volume Units in Chapter 2C, Code Tables, for valid entries.
4.17.2.8 BUI-8 Container Catalog Number (ST) 03380
Definition: The string catalog number of the blood unit container, which includes a specific container code to identify a collection bag.
4.17.2.9 BUI-9 Container Lot Number (ST) 03381
Definition: The lot number for the collection bag container as assigned by the container manufacturer.
4.17.2.10 BUI-10 Container Manufacturer (XON) 03382
Components: <Organization Name (ST)> ^ <Organization Name Type Code (CWE)> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <WITHDRAWN Constituent> ^ <Assigning Authority (HD)> ^ <Identifier Type Code (ID)> ^ <Assigning Facility (HD)> ^ <Name Representation Code (ID)> ^ <Organization Identifier (ST)>
Subcomponents for Organization Name Type Code (CWE): <Identifier (ST)> & <Text (ST)> & <Name of Coding System (ID)> & <Alternate Identifier (ST)> & <Alternate Text (ST)> & <Name of Alternate Coding System (ID)> & <Coding System Version ID (ST)> & <Alternate Coding System Version ID (ST)> & <Original Text (ST)> & <Second Alternate Identifier (ST)> & <Second Alternate Text (ST)> & <Name of Second Alternate Coding System (ID)> & <Second Alternate Coding System Version ID (ST)> & <Coding System OID (ST)> & <Value Set OID (ST)> & <Value Set Version ID (DTM)> & <Alternate Coding System OID (ST)> & <Alternate Value Set OID (ST)> & <Alternate Value Set Version ID (DTM)> & <Second Alternate Coding System OID (ST)> & <Second Alternate Value Set OID (ST)> & <Second Alternate Value Set Version ID (DTM)>
Subcomponents for Assigning Authority (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Subcomponents for Assigning Facility (HD): <Namespace ID (IS)> & <Universal ID (ST)> & <Universal ID Type (ID)>
Definition: The organization which manufactured the collection bag container.
4.17.2.11 BUI-11 Transport Temperature (NR) 03383
Components: <Low Value (NM)> ^ <High Value (NM)>
Definition: The temperature range between which the blood unit must be kept during transport.
4.17.2.12 BUI-12 Transport Temperature Units (CNE) 03384
Components: <Identifier (ST)> ^ <Text (ST)> ^ <Name of Coding System (ID)> ^ <Alternate Identifier (ST)> ^ <Alternate Text (ST)> ^ <Name of Alternate Coding System (ID)> ^ <Coding System Version ID (ST)> ^ <Alternate Coding System Version ID (ST)> ^ <Original Text (ST)> ^ <Second Alternate Identifier (ST)> ^ <Second Alternate Text (ST)> ^ <Name of Second Alternate Coding System (ID)> ^ <Second Alternate Coding System Version ID (ST)> ^ <Coding System OID (ST)> ^ <Value Set OID (ST)> ^ <Value Set Version ID (DTM)> ^ <Alternate Coding System OID (ST)> ^ <Alternate Value Set OID (ST)> ^ <Alternate Value Set Version ID (DTM)> ^ <Second Alternate Coding System OID (ST)> ^ <Second Alternate Value Set OID (ST)> ^ <Second Alternate Value Set Version ID (DTM)>
Definition: The unit of measure of the transport temperature range. Concepts are pulled from the UCUM code system (www.unitsofmeasure.org). Refer to HL7-Defined Table 0931 - Temperature Units in Chapter 2C, Code Tables, for valid entries.
4.18 TABLES LISTINGS
4.18.1 Figure 4-8 Associations between Order Control Codes and Trigger Events
Figure 4-8 defines the explicit relationships that exist between Order Control Codes and Trigger Events. A value of "Y" at the intersection of an Order Control Code and a Trigger Event indicates that is a valid combination that can be used in a message. A value of "N" indicates that combination is not valid in any message. No value at an intersection indicates that no business case has been brought forward for to justify or exclude that combination. Implementers are encouraged to bring business cases forward for currently undefined combinations of Order Control Codes and Trigger Events.
Figure 4-8 Order Control Codes / Trigger Event Matrix
O01
O02
O03
O04
O05
O06
O07
O08
O09
O10
O11
O12
O13
O14
O15
O16
O18
O19
O20
O21
P03
P11
Q06
R01
AF
Y
Y
CA
Y
Y
Y
Y
Y
Y
Y
CH
Y
Y
Y
Y
Y
Y
Y
CN
Y
CR
Y
Y
Y
Y
Y
Y
DC
Y
Y
Y
Y
Y
Y
Y
DE
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
DF
Y
Y
Y
DR
Y
Y
Y
Y
Y
Y
FU
Y
Y
HD
Y
Y
Y
Y
Y
HR
Y
Y
Y
Y
Y
Y
LI
Y
Y
Y
Y
Y
Y
Y
MC
Y
Y
NA
Y
Y
Y
Y
Y
NW
Y
Y
Y
Y
Y
Y
Y
OC
Y
Y
Y
Y
Y
Y
Y
Y
OD
Y
Y
Y
Y
Y
Y
Y
Y
OE
Y
Y
Y
Y
Y
Y
Y
Y
OF
Y
Y
OH
Y
Y
Y
Y
Y
Y
Y
Y
OK
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
OP
Y
OR
Y
Y
Y
Y
Y
Y
PA
Y
Y
Y
Y
Y
Y
Y
Y
PR
Y
Y
Y
PY
Y
RE
Y
Y
Y
Y
Y
Y
Y
RF
Y
Y
Y
RL
Y
Y
Y
Y
Y
Y
Y
RO
Y
Y
Y
Y
Y
Y
Y
RP
Y
Y
Y
Y
Y
Y
RQ
Y
Y
Y
Y
Y
RR
Y
RU
Y
Y
Y
Y
Y
Y
SC
Y
Y
Y
SN
Y
Y
Y
Y
Y
SR
Y
Y
SS
Y
Y
Y
UA
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
UC
Y
Y
Y
Y
Y
Y
UD
Y
Y
Y
Y
Y
Y
UF
Y
Y
UH
Y
Y
Y
Y
Y
Y
UM
Y
Y
Y
Y
Y
UN
Y
Y
Y
Y
Y
Y
Y
Y
UR
Y
Y
Y
Y
Y
Y
UX
Y
Y
Y
Y
Y
Y
XO
Y
Y
Y
Y
Y
Y
Y
XR
Y
Y
Y
Y
Y
Y
XX
Y
Y
Y
Y
Y
Y
Y
Y
Editor's note: The order control codes need to be assessed for their application to these trigger events O22 through O48. The current table structure will not accommodate these additional columns; a new table structure needs to be considered.
4.19 OUTSTANDING ISSUES
In approving the transfusion service messages and related segments for their initial inclusion in version 2.5, it was noted that the messages do not support information relative to DNA and/or RNA extracts of blood and/or blood products. Future consideration of this is dependent upon the development of related use cases to define requirements.
At present, ORC-34 Order Workflow Profile in section 4.5.1.34, does not contain a reference to a valid User-Defined Table.









